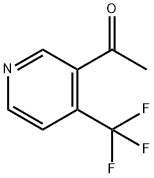
1-[4-(Trifluoromethyl)-3-pyridinyl]-ethanone synthesis
- Product Name:1-[4-(Trifluoromethyl)-3-pyridinyl]-ethanone
- CAS Number:955997-27-0
- Molecular formula:C8H6F3NO
- Molecular Weight:189.13
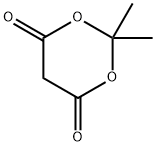
2033-24-1
628 suppliers
$6.00/25g
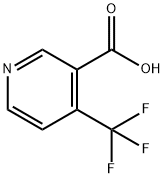
158063-66-2
350 suppliers
$5.00/250mg
![1-[4-(Trifluoromethyl)-3-pyridinyl]-ethanone](/CAS/20200331/GIF/955997-27-0.gif)
955997-27-0
19 suppliers
inquiry
Yield:955997-27-0 62%
Reaction Conditions:
with PyBOP;triethylamine in tetrahydrofuran at 20; for 18 h;
Steps:
1
In a 250 mL round bottom flask was placed 3.0 g of 4-trifluoronicotinic acid (15.7 mmol, 1 eq) in 100 mL of THF. To this was added 5.3 mL (3.8 g, 37.7 mmol, 2.4 eq) of triethylamine and 9.8 g (18.8 mmol, 1.2 eq) of [PYBOP.] This was allowed to stir for 10 min at room temperature where 2.7 g of [MELDRUM'S] acid (18.8 mmol, 1.2 eq) was added and the reaction allowed stirring at room temperature overnight. (18 h) At this point, 30 mL of 1 M HCI (aq) was added and the reaction turned immediately from orange to purple. This was then heated at for 18 h gradually turning from purple to yellow. The reaction was then basified with saturated [NAHCO3] and extracted with EtOAc (3 x 200 mL). The combined organic layers were dried, filtered, and evaporated. The residue was purified via BIOTAGE (35% EtOAc/Hex) to provide methyl [4-TRIFLUOROMETHYLNICOTINATE] 1.84 g (62%) of the desired product as a colorless oil. TLC Rf= 0.57 (50% EtOAc: Hex).
References:
WO2004/7502,2004,A1 Location in patent:Page 20

2033-24-1
628 suppliers
$6.00/25g
![1-[4-(Trifluoromethyl)-3-pyridinyl]-ethanone](/CAS/20200331/GIF/955997-27-0.gif)
955997-27-0
19 suppliers
inquiry