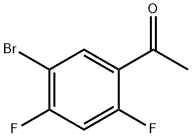
1-(5-Bromo-2,4-difluoro-phenyl)-ethanone synthesis
- Product Name:1-(5-Bromo-2,4-difluoro-phenyl)-ethanone
- CAS Number:864773-64-8
- Molecular formula:C8H5BrF2O
- Molecular Weight:235.03
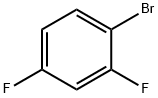
348-57-2

75-36-5

864773-64-8
1. in a dry reaction flask, anhydrous AlCl3 (1040 g, 7878.7 mmol) was added to 1-bromo-2,4-difluorobenzene (600 g, 3108.9 mmol) and stirred at 60 °C for 10 min. 2. acetyl chloride (366 g, 4662.4 mmol) was added slowly dropwise to the above mixture, maintaining the temperature at 60 °C for 4 hours. 3. after the dropwise addition was completed, the reaction mixture was continued to be stirred at 60 °C for 6 hours. 4. the reaction mixture was cooled to -10 °C and ice (2450 g) was added slowly, this process was controlled to be completed within 1.5 hours. 5. 12N HCl (1500mL) was added and stirred for 1 hour at room temperature. 6. EtOAc (9000mL) was added for extraction, the organic layer was separated, washed with water, dried over anhydrous Na2SO4 and filtered. 7. The organic phase was concentrated under pressure and the resulting residue was purified by silica gel column chromatography (eluent: petroleum ether/EtOAc, 50/1) to afford the target product 1-(5-bromo-2,4-difluorophenyl)ethanone (300 g, 41% yield).

348-57-2
424 suppliers
$5.00/5g

75-36-5
588 suppliers
$17.92/100G

864773-64-8
98 suppliers
$9.00/100mg
Yield: 41%
Reaction Conditions:
Stage #1:2,4-difluorobromobenzene;acetyl chloride with aluminum (III) chloride at 60 - 95; for 10 h;Friedel Crafts Acylation;
Stage #2: at -10; for 1 h;
Steps:
A19
Example A19Preparation of intermediate 19: l-(5-bromo-2,4-difluoro-phenyl)-ethanoneA mixture of A1C13 (200 g, 1515.1 mmol) in l-bromo-2,4-difluoro-benzene (120 g, 621.79 mmol) was stirred at 60 °C for 10 minutes. Then, acetyl chloride (73 g,929.9 mmol) was added dropwise over 4 hours and the mixture stirred at 95 °C for 6 hours. The mixture was cooled at - 10 °C and ice (300 g) was added over 1 hour. Then, AcOEt was added (500 mL) and the separated organic layer was washed with water, dried (Na2S04), filtered and concentrated in vacuo. The residue was purified by flash column chromatography (silica gel; AcOEt in heptane 1/50). The desired fractions were collected and concentrated in vacuo to yield intermediate 19 (60 g, 41% yield).
References:
JANSSEN PHARMACEUTICA NV;TRABANCO-SUÁREZ, Andrés, Avelino;TRESADERN, Gary, John;MACDONALD, Gregor, James;VEGA RAMIRO, Juan, Antonio WO2011/154374, 2011, A1 Location in patent:Page/Page column 26
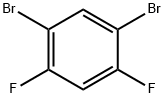
28342-75-8
141 suppliers
$6.00/1g
78191-00-1
286 suppliers
$6.00/5g

864773-64-8
98 suppliers
$9.00/100mg