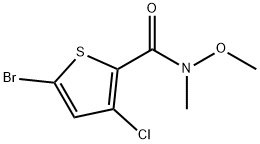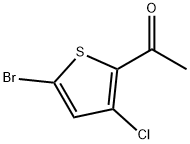
1-(5-Bromo-3-chlorothiophen-2-yl)ethanone synthesis
- Product Name:1-(5-Bromo-3-chlorothiophen-2-yl)ethanone
- CAS Number:1403468-44-9
- Molecular formula:C6H4BrClOS
- Molecular Weight:239.52
78191-00-1
290 suppliers
$6.00/5g
77893-68-6
74 suppliers
$35.00/100mg

1403468-44-9
8 suppliers
inquiry
Yield:1403468-44-9 100%
Reaction Conditions:
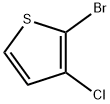
Stage #1: 2-bromo-3-chlorothiophenewith n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 0.5 h;
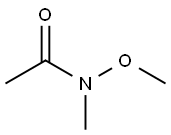
Stage #2: N-Methoxy-N-methylacetamide in tetrahydrofuran at -78; for 0.5 h;
Steps:
Preparation 62 l-(5-bromo-3-chlorothiophen-2-yl)ethanone
Preparation 62 l-(5-bromo-3-chlorothiophen-2-yl)ethanone To a solution of diisopropylamine (6.62 ml, 46.4 mmol) in THF (57.2 ml) at -78 °C was added a solution of BuLi (18.57 ml, 46.4 mmol) dropwise. When the addition was complete, the reaction mixture was allowed to stir at -78 °C for an additional 30 min. A solution of 2-bromo-3-chlorothiophene (Preparation 61, 9.17 g, 46.4 mmol) in THF (1 1.43 ml) was then added rapidly in one aliquot. The resulting mixture was stirred at -78 °C for 30 min. A solution of N-methoxy-N-methylacetamide (1.915 g, 18.57 mmol) in THF (5.72 ml) was then added dropwise to the reaction mixture, which was then allowed to stir for an additional 30 min at -78 °C. The reaction mixture was then quenched by the slow addition of IN HC1 (120 mL). After coming to rt, the mixture was extracted with ether (3 x 50 mL). The combined extracts were washed with water (50 mL), dried over MgS04, filtered and concentrated in vacuo. Purification by flash chromatography (silica, EtOAc/Hexanes) gave l-(5-bromo-3-chlorothiophen-2-yl)ethanone (100% yield); XH NMR (500MHz, CHLOROFORM-d) δ 7.03 (s, 1H), 3.86 (t, J=6.2 Hz, 1H), 3.50 (t, J=6.3 Hz, 1H), 2.65 (s, 3H).
References:
WO2014/98831,2014,A1 Location in patent:Page/Page column 64; 65
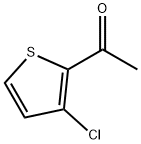
89581-82-8
76 suppliers
$31.00/1g

1403468-44-9
8 suppliers
inquiry
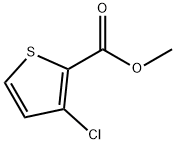
88105-17-3
137 suppliers
$8.00/1g

1403468-44-9
8 suppliers
inquiry
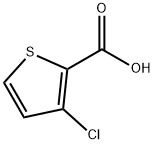
59337-89-2
208 suppliers
$5.00/250mg

1403468-44-9
8 suppliers
inquiry