
1-(5-broMopyridin-2-yl)cyclopropanaMine synthesis
- Product Name:1-(5-broMopyridin-2-yl)cyclopropanaMine
- CAS Number:944718-22-3
- Molecular formula:C8H9BrN2
- Molecular Weight:213.07
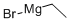
925-90-6
349 suppliers
$12.00/5ml
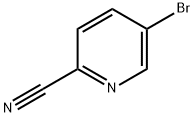
97483-77-7
575 suppliers
$10.00/5g

944718-22-3
23 suppliers
inquiry
Yield:944718-22-3 30%
Reaction Conditions:
Stage #1: ethylmagnesium bromide;2-Cyano-5-bromopyridine;titanium(IV) isopropylate in diethyl ether at -78 - 20; for 1.58333 h;
Stage #2: with boron trifluoride diethyl etherate in diethyl ether; for 2 h;
Steps:
414.A
Part A: Compound 414 (1.0 g, 5.46 mmol) was suspended in diethylether (30 mL) and cooled to -78 0C. Titanium (IV) isopropoxide (1.7 mL, 6.01 mmol) was added dropwise and stirred for 5 minutes. Ethyl magnesium bromide (3M in diethylether, 4.0 mL) was added dropwise and stirred for 30 minutes at the same temperature and 1 hour at room temperature. Boron trifluoride dietherate (1.55 g, 10.92 mmol) was added dropwise and the reaction was stirred for 2 hours. The reaction was quenched with 1 M hydrochloric acid and the aqueous layer was washed with diethyl ether. The aqueous layer was made basic (pH = 10) and extracted with ethyl acetate. The organic layer was dried over sodium sulfate and concentrated. Column chromatography (1 :1 hexanes/ethyl acetate) provided the desired product (350 mg, 30%). 1H NMR (400 MHz, CDCI3) δ 8.5 (d, 1 H), 7.7 (m, 1H)1 7.3 (d, 1 H), 1.25 (m, 2H), 1.15 (m, 2H).
References:
WO2007/84451,2007,A1 Location in patent:Page/Page column 148
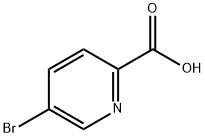
30766-11-1
407 suppliers
$3.00/1g

944718-22-3
23 suppliers
inquiry
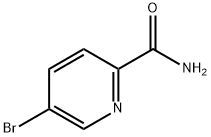
90145-48-5
121 suppliers
$31.00/250mg

944718-22-3
23 suppliers
inquiry