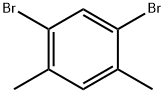
1,5-Dibromo-2,4-dimethylbenzene synthesis
- Product Name:1,5-Dibromo-2,4-dimethylbenzene
- CAS Number:615-87-2
- Molecular formula:C8H8Br2
- Molecular Weight:263.96
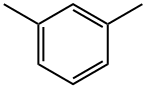
108-38-3

615-87-2
The general procedure for the synthesis of 2,4-dimethyl-m-dibromobenzene from m-xylene was as follows: 7.5 mmol (0.795 g) of m-xylene, 33.6 mmol (7.56 g) of zinc bromide, and 60 mL of nitromethane were added to a 200 mL aubergine flask. The resulting solution was stirred at room temperature, followed by the slow addition of 15.8 mmol (6.19 g) di-n-butyltetrabutylammonium in batches. The reaction mixture was stirred continuously for 44 hours at room temperature before the reaction was quenched by the addition of aqueous sodium sulfite and extracted with ether. The organic layers were combined and concentrated under reduced pressure to give a yellow solid. Hexane was added to the yellow solid and the mixture was heated to about 85°C and filtered. The filtrate was concentrated under reduced pressure to give 0.945 g of white solid, the target product 2,4-dimethyl m-dibromobenzene (yield: 48%).
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

108-38-3
383 suppliers
$10.60/50gm:

615-87-2
95 suppliers
$9.00/250mg
Yield: 48.6%
Reaction Conditions:
Stage #1:m-xylene with iodine for 1 h;Cooling with ice;
Stage #2:bromine
Steps:
1,5-Dibromo-2,4-dimethylbenzene
A 100-mL round bottomed flask wrapped in aluminum foil is charged with m-xylene (40.0 mL, 328 mmol) and iodine (0.48 g, 1.90 mmol). The mixture is stirred for about an hour while cooling in an ice bath. Bromine (34 mL, 656 mmol) is added via dropping funnel over a period of one hour. After overnight reaction, potassium hydroxide (KOH) (20% aq, 150 mL) is added and the resulting mixture is heated gently using a heating mantle. The solid is melted and the biphasic mixture is stirred for about one hour as the yellow color slowly fades. After cooling, the liquid is decanted and the white solid is collected by filtration and washed with additional water (3*100 mL), then recrystallized from ethanol (250 mL). Yield=42.0 g (48.6%)
References:
Dow Global Technologies, LLC;Kuhlman, Roger L.;Whiteker, Gregory T. US8592615, 2013, B2 Location in patent:Sheet 27

108-38-3
383 suppliers
$10.60/50gm:

615-87-2
95 suppliers
$9.00/250mg

108-38-3
383 suppliers
$10.60/50gm:
583-70-0
241 suppliers
$15.00/5mg

615-87-2
95 suppliers
$9.00/250mg

108-38-3
383 suppliers
$10.60/50gm:

615-87-2
95 suppliers
$9.00/250mg
90434-19-8
51 suppliers
$11.00/1g
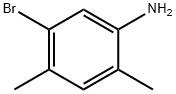
69383-60-4
70 suppliers
$19.00/250mg

615-87-2
95 suppliers
$9.00/250mg