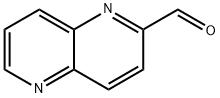
1,5-NAPHTHYRIDINE-2-CARBOXALDEHYDE synthesis
- Product Name:1,5-NAPHTHYRIDINE-2-CARBOXALDEHYDE
- CAS Number:883864-92-4
- Molecular formula:C9H6N2O
- Molecular Weight:158.16
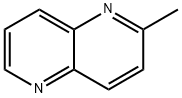
7675-32-3
97 suppliers
$26.00/100mg

883864-92-4
27 suppliers
inquiry
Yield: 48%
Reaction Conditions:
with selenium(IV) oxide for 2 h;Reflux;
Steps:
A 100 mL round bottom flask equipped with a condenser (with a calcium sulfate drying tube) and a magnetic stirbar was charged with 2-methyl-1,5-naphthyridine (CAS7675-32-3, Synchem-OHG CDP146FP1, 433 mg, 3.0 mmol). Dioxane (10 mL) was added to give a stirred solution, then selenium dioxide (366 mg, 3.3 mmol) was added and the reaction mixture was heated to reflux with an oil bath. The stirred reaction mixture was maintained at reflux for 2 h, then cooled to ambient temperature and filtered through diatomaceous earth. The filtrate was concentrated under reduced pressure and the residue was purified by column chromatography on an Analogix IF-280 (Varian SF25-60 g, 100:0 to 0:100 hexane/EtOAc). Fractions 10-17 were combined and concentrated under reduced pressure to give a white solid that was dried overnight in a vacuum oven at ambient temperature. The dried solid Intermediate 4B, 1,5-naphthyridine-2-carbaldehyde weighed 229 mg (48%). 1H NMR (300 MHz, CDCl3) δ 10.25 (d, J=0.9 Hz, 1H), 9.12 (dd, J=4.2, 1.6 Hz, 1H), 8.60-8.54 (m, 2H), 8.28 (d, J=8.7 Hz, 1H), 7.77 (dd, J=8.6, 4.2 Hz, 1H). MS (DCI-NH3) m/z=159 (M+H)+, m/z=176 (M+NH4)+.
References:
AbbVie Inc.;Black, Lawrence A. US2013/343992, 2013, A1 Location in patent:Paragraph 0260