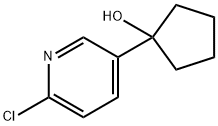
1-(6-chloro-pyridin-3-yl)cyclopentanol synthesis
- Product Name:1-(6-chloro-pyridin-3-yl)cyclopentanol
- CAS Number:1011473-96-3
- Molecular formula:C10H12ClNO
- Molecular Weight:197.66
53939-30-3
452 suppliers
$5.00/1g

120-92-3
392 suppliers
$11.00/25g

1011473-96-3
2 suppliers
inquiry
Yield:1011473-96-3 76%
Reaction Conditions:
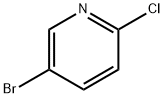
Stage #1: 5-bromo-2-chloropyridinewith n-butyllithium in diethyl ether;hexane; for 1 h;Cooling with acetone-dry ice;
Stage #2: cyclopentanone in diethyl ether;hexane at -20;
Steps:
III.A
(A) 5-Bromo-2-chloropyridine (3.0 g, 15.6 mmol) was dissolved in diethyl ether (100 mL) in an oven-dried, nitrogen-flushed 250 mL round bottomed flask and cooled in a dry ice/acetone bath under nitrogen. n-BuLi (1.05 g, 16.4 mmol, 6.6 mL of a 2.5 M solution in hexanes) was added via syringe and the orange heterogenous mixture allowed to stir for 1 hour. Cyclopentanone (1.3 g, 15.6 mmol) was added via syringe and the mixture allowed to warm to -20° C. before being quenched with 1N HCl. The mixture was extracted with EtOAc and the organic extract washed with sat. NaHCO3. The NaHCO3 wash was used to neutralize the first aqueous layer and the combined aqueous layers were extracted with additional EtOAc and the combined organic layers dried (Na2SO4), filtered, concentrated and purified by flash silica gel chromatography (hexanes:EtOAc; 2:1) to give 1-(6-chloro-pyridin-3-yl)cyclopentanol (2.33 g, 76%) as an off white solid: mp 92-93° C., LC/MS (ESI) m/z 197, 1H NMR (300 MHz, CDCl3) δ 8.50 (d, 1H, J=2.4 Hz), 7.77 (dd, 1H, J=8.4, 2.4 Hz), 7.28 (d, 1H, J=8.4 Hz), 2.05-1.80 (m, 8H), 1.60 (s, 1H, OH).
References:
US2008/108666,2008,A1 Location in patent:Page/Page column 10