
1-(6-ETHYNYL-PYRIDIN-2-YL)-ETHANONE synthesis
- Product Name:1-(6-ETHYNYL-PYRIDIN-2-YL)-ETHANONE
- CAS Number:874379-35-8
- Molecular formula:C9H7NO
- Molecular Weight:145.158
![Ethanone, 1-[6-[2-(trimethylsilyl)ethynyl]-2-pyridinyl]-](/CAS/20210305/GIF/874379-34-7.gif)
874379-34-7
1 suppliers
inquiry

874379-35-8
19 suppliers
inquiry
Yield:874379-35-8 402 mg
Reaction Conditions:
with methanol;potassium carbonate at 20; for 2 h;
Steps:
Method M. l-(6-ethyny]pyridin-2-yl)ethan-l-one
Method M. l-(6-ethyny]pyridin-2-yl)ethan-l-one l-(6-bromopyridin-2-yl)ethan-l-one (l .Og. 5.0mmol, leq), Cul (38mg, 0.2mmol, 0.04 eq), PdCl2(PPh3)2 (140mg, 0.2mmol, 0.04 eq) in THF (10ml), TEA (1.5ml) was degassed under bubbling nitrogen for 10 min. To this solution was added TMS-acetylene (1.42 ml, 10 mmol, 2 eq) and the reaction was stirred for 2 hours at RT. The reaction was diluted in hexanes and filtered over a plug of silica gel to remove the majority of impurities. The eluent was concentrated under reduced pressure and further purified by silica gel chromatography (5% EtOAc/Hexanes) to afford l-(6-((trimethylsilyl)ethynyl)pyridin-2-yl)ethan-l-one (1.09g) as a yellow oil that was used without further purification. To a solution of l-(6- ((trimethylsilyl)ethynyl)pyridin-2-yl)ethan-l -one (crude from previous reaction) in methanol (20ml) was added a large excess of potassium carbonate. The reaction was stirred 2 hours at RT and concentrated under reduced pressure. The concentrate was partitioned in DCM/water, extracted 2 x DCM, dried over sodium sulfate and concentrated. The crude product was purified by silica gel chromatography to give l-(6-ethynylpyridin-2-yl)ethan-l -one (402 mg, 55% yield over 2 steps) as a white solid.
References:
WO2016/123253,2016,A1 Location in patent:Page/Page column 32
![Ethanone, 1-[6-(3-hydroxy-3-methyl-1-butyn-1-yl)-2-pyridinyl]-](/CAS/20210305/GIF/874379-36-9.gif)
874379-36-9
0 suppliers
inquiry

874379-35-8
19 suppliers
inquiry
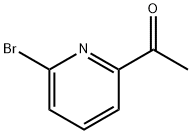
49669-13-8
227 suppliers
$5.00/250mg

874379-35-8
19 suppliers
inquiry
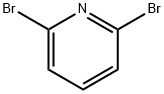
626-05-1
505 suppliers
$9.00/10g

874379-35-8
19 suppliers
inquiry