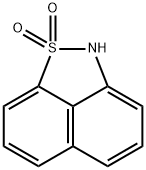
1,8-NAPHTHOSULTAM synthesis
- Product Name:1,8-NAPHTHOSULTAM
- CAS Number:603-72-5
- Molecular formula:C10H7NO2S
- Molecular Weight:205.23
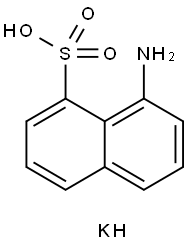
16619-06-0

603-72-5
General procedure for the synthesis of 1,8-naphthalenesulfonolactam from 1,8-naphthalenesulfonic acid potassium salt (CAS: 16619-06-0): 5 g of 1,8-naphthalenesulfonic acid potassium salt was pulverized and placed in a flask containing 15 g of POCl3. After installation of a reflux condenser, the reaction system is heated in an oil bath to 130°C. When the temperature reaches 100°C, HCl gas starts to be released, which has to be discharged through the flue. To facilitate the reaction, the flask was shaken periodically. After the release of irritating gases stops, the heating is continued until the reaction mixture no longer produces dye in the diazotization and color development test, usually for a reaction time of 3 hours. Due to the slurry-like consistency of the reaction mixture, caution is required to remove excess POCl3. Subsequently, the reaction mixture is quenched by slowly pouring it into an ice bath. The gray solid product was collected by diafiltration and washed repeatedly with water to remove most of the phosphoric acid. The crude product was dried and purified by recrystallization with benzene. Product melting point: 175-180°C; 1H NMR (DMSO-d6, 300 MHz) δ: 8.62 (d, 1H, J = 8.0 Hz), 8.12 (d, 1H, J = 8.0 Hz), 7.75-7.74 (m, 1H), 7.61 (d, 1H, J = 8.0 Hz), 7.32 (dd, 1H, J = 7.5, 6.7 Hz), 6.7 Hz), 6.7 Hz). 6.7 Hz), 6.90 (d, 1H, J = 6.8 Hz), 5.36 (s, 1H); MS (EI): m/z 206 (M++H).

16619-06-0
0 suppliers
inquiry

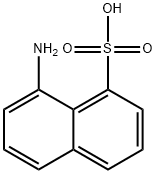
603-72-5
54 suppliers
$127.00/5g
Yield:603-72-5 70%
Reaction Conditions:
with trichlorophosphate at 130; for 3 h;
Steps:
5.3. 1,2-Dihydro-1λ6-naphtho[1,8-cd]isothiazole-1,1-dione 6
Five grams of the potassium salt of 1,8 naphthyl amine sulphonic acid were pulverized and placed in a flask containing three times that weight of POCl3. After attaching a reflux condenser the whole was heated in an oil bath to 130 °C. At 100 °C the offensive vapors of hydrochloric acid are given off and must be led into flue. To hasten the reaction the flask is shaken repeatedly. After the irritating vapors have ceased to be given off the heating is continued until the contents of the flask ceases to form a dyestuff when diazotized and developed. As a rule the reaction is complete after 3 h. The removal of the excess POCl3 requires some care because of the mud like consistency of the contents of the flask. Finally the contents of the flask are poured into ice bath. The grey product is filtered off by means of a suction pump and greater part of the phosphoric acid removed by repeated washing. The crude product was recrystallised from benzene after through drying. Mp 175-180 °C; 1H NMR (DMSO, 300 MHz): δ 8.62 (d, 1H, J = 8.0 Hz), 8.12 (d, 1H, J = 8.0 Hz), 7.75-7.74 (m, 1H), 7.61 (d, 1H, J = 8.0 Hz), 7.32 (dd, 1H, J = 7.5, 6.7 Hz), 6.90 (d, 1H, J = 6.8 Hz), 5.36 (s, 1H); MS (EI): m/z 206 (M++H).
References:
Kamal, Ahmed;Ramakrishna;Lakshma Nayak;Raju;Subba Rao;Viswanath;Vishnuvardhan;Ramakrishna, Sistla;Srinivas [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 2,p. 789 - 800] Location in patent:experimental part
82-75-7
280 suppliers
$7.00/25g

603-72-5
54 suppliers
$127.00/5g
![Ethanone, 1-(1,1-dioxido-2H-naphth[1,8-cd]isothiazol-2-yl)-](/CAS/20210305/GIF/116082-56-5.gif)