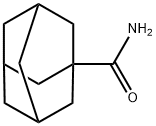
1-ADAMANTANECARBOXAMIDE synthesis
- Product Name:1-ADAMANTANECARBOXAMIDE
- CAS Number:5511-18-2
- Molecular formula:C11H17NO
- Molecular Weight:179.26
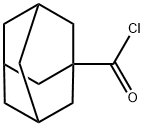
2094-72-6

5511-18-2
GENERAL STEPS: To a stirred mixture of 1-adamantanecarboxylic acid (11, 36.05 g, 200 mmol) and DMF (5 drops) in CH2Cl2 (360 mL) was added thionyl chloride (26.17 g, 220 mmol) in batches under cooling conditions in an ice-water bath. The resulting mixture was refluxed until the release of HCl gas ceased (usually takes 2 hours). Subsequently, the solvent and excess thionyl chloride were removed using a rotary evaporator and the crude product, adamantanoyl chloride (12), was dissolved in THF (100 mL). This solution was slowly added dropwise to concentrated ammonia (400 mL) cooled and stirred in an ice water bath. After the dropwise addition was completed, the white slurry formed was continued to be stirred at room temperature for 2 hours. The white precipitate was collected by vacuum filtration, washed with cold hexane, and dried under vacuum to give 1-adamantanecarboxamide (13). The product was a white solid with a yield of 34.06 g (95% yield) and a melting point of 177-179 °C (literature value: 185.5-191.7 °C [48]).1H-NMR (DMSO-d6, 400 MHz) δ: 6.89 (brs, 1H), 6.62 (brs, 1H), 1.93 (s, 3H), 1.73-1.74 (m , 6H), 1.60-1.68 (m, 6H).

2094-72-6
200 suppliers
$15.00/5g

5511-18-2
160 suppliers
$10.00/250mg
Yield: 95%
Reaction Conditions:
with ammonia in water at 20; for 2 h;
Steps:
.2.1. General Procedure for the Synthesis of Substituted Methylamines 2d, 2k and 2m
To a stirred mixture of 1-adamantanecarboxylic acid (11, 36.05 g, 200 mmol) and DMF (5 drops)in CH2Cl2 (360 mL) cooled in an ice-water bath was added thionyl chloride (26.17 g, 220 mmol) inone portion. The resulting mixture was refluxed until the evolution of HCl gas ceased (typicallywithin 2 h). The solvent and excess thionyl chloride were then removed on a rotary evaporator, andthe crude acid chloride 12 was dissolved in THF (100 mL). The resulting solution was then slowlyadded dropwise to stirred concentrated aqueous ammonia (400 mL) cooled in an ice-water bath.After addition, the resulting white slurry was stirred at room temperature for another 2 h, and thewhite precipitates were collected via vacuum filtration, washed with cold n-hexane and dried invacuo to afford 1-adamantanecarboxamide (13). White solid; 34.06 g (95%); m.p. 177-179 °C(literature value, 185.5-191.7 °C [48]). 1H-NMR (DMSO-d6, 400 MHz) δ: 6.89 (brs, 1H), 6.62 (brs, 1H),1.93 (s, 3H), 1.73-1.74 (m, 6H), 1.60-1.68 (m, 6H).
References:
Cai, Wenqing;Wu, Jingwei;Liu, Wei;Xie, Yafei;Liu, Yuqiang;Zhang, Shuo;Xu, Weiren;Tang, Lida;Wang, Jianwu;Zhao, Guilong [Molecules,2018,vol. 23,# 2,art. no. 252]
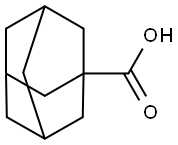
828-51-3
431 suppliers
$10.00/5g

5511-18-2
160 suppliers
$10.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5511-18-2
160 suppliers
$10.00/250mg
23074-42-2
137 suppliers
$21.21/1gm:

5511-18-2
160 suppliers
$10.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5511-18-2
160 suppliers
$10.00/250mg