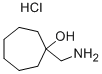
1-(AMINOMETHYL)-CYCLOHEPTANOL HYDROCHLORIDE synthesis
- Product Name:1-(AMINOMETHYL)-CYCLOHEPTANOL HYDROCHLORIDE
- CAS Number:2815-39-6
- Molecular formula:C8H18ClNO
- Molecular Weight:179.69
![Cycloheptanecarbonitrile, 1-[(trimethylsilyl)oxy]-](/CAS/20180601/GIF/91390-82-8.gif)
91390-82-8
3 suppliers
inquiry

2815-39-6
29 suppliers
inquiry
Yield:2815-39-6 1.05 g
Reaction Conditions:
Stage #1: 1-((trimethylsilyl)oxy)cycloheptane-1-carbonitrilewith lithium aluminium tetrahydride in tetrahydrofuran;diethyl ether at 0 - 20; for 2 h;
Stage #2: with hydrogenchloride in 1,4-dioxane;diethyl ether at 20;
Steps:
45.2 45.2 1-Aminomethyl-cycloheptanol hydrochloride
45.2
1-Aminomethyl-cycloheptanol hydrochloride
A 2M solution of lithium aluminum hydride (6.13 mL) in THF was diluted with anh. Et2O (6.5 mL) and a solution of 1-trimethylsilanyloxy-cycloheptanecarbonitrile (1.73 g) in anh. Et2O (3.3 mL) was added dropwise for 15 min at 0° C.
The ice bath was removed and the reaction mixture was stirred for 2 h at RT.
It was cooled to 0° C. and quenched successively with ice H2O (1 mL), a 1M solution of NaOH (1 mL) and ice H2O (3.3 mL).
The mixture was stirred for 10 min at RT, diluted with Et2O and filtered over a pad of celite.
The filtrate was concentrated in vacuo and the residue was redissolved in Et2O (13 mL) and few drops of dioxane.
A 4M solution of hydrogen chloride in dioxane (6.6 mL) was added at RT and the precipitate was filtered to give 1.05 g of the 1-aminomethyl-cycloheptanol hydrochloride as a white solid.
1H NMR (CD3OD) δ: 2.90 (s, 2H), 1.59 (m, 12H)
13C NMR (CD3OD) δ: 72.1, 47.7, 38.1, 29.6, 21.7
References:
US2014/73651,2014,A1 Location in patent:Paragraph 0523; 0524; 0525
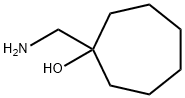
45732-95-4
50 suppliers
$65.00/25mg

2815-39-6
29 suppliers
inquiry
502-42-1
210 suppliers
$10.00/1g

2815-39-6
29 suppliers
inquiry