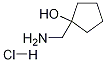
1-(aminomethyl)cyclopentanol hydrochloride synthesis
- Product Name:1-(aminomethyl)cyclopentanol hydrochloride
- CAS Number:76066-27-8
- Molecular formula:C6H14ClNO
- Molecular Weight:151.6345
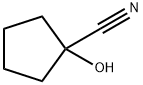
5117-85-1
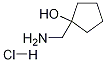
76066-27-8
The general procedure for the synthesis of 1-(aminomethyl)cyclopentanol hydrochloride from 1-hydroxycyclopentanecarbonitrile was as follows: to a mixture of cyclopentanone (5.50 g, 65.0 mmol) and ZnBr2 (0.20 g, 8.00 mmol) under cooling in an ice bath, TMSCN (10.0 mL, 73.4 mmol) was slowly added, and the reaction was subsequently stirred at room temperature for mixture for 12 hours. The resulting cyanohydrin solution was slowly added dropwise to a suspension of LiAlH4 (8.34 g, 219 mmol) in THF (30.0 mL) at 0 °C, and the mixture was then heated to reflux for 1 hour. Upon completion of the reaction, the mixture was cooled to room temperature and water (10.0 mL), 4M NaOH aqueous solution (10.0 mL), and water (10.0 mL) were added slowly and sequentially under vigorous stirring. Insoluble material was removed by filtration through a diatomaceous earth pad, and the organic phase was separated and dried with KOH. The dried organic phase was decanted, dried over Na2SO4, filtered and concentrated. The residue was dissolved in MTBE (100 mL), a dioxane solution (10.0 mL) of 4N HCl was added, and stirred at room temperature for 30 minutes. The resulting white solid precipitate was collected by filtration through a sintered funnel to afford the target product 1-(aminomethyl)cyclopentanol hydrochloride (6.68 g, 68% yield).

5117-85-1
36 suppliers
inquiry
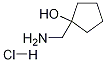
76066-27-8
45 suppliers
$44.00/250mg
Yield:76066-27-8 68%
Reaction Conditions:
Stage #1:1-hydroxycyclopentanecarbonitrile with lithium aluminium tetrahydride in tetrahydrofuran at 0; for 1 h;Reflux;
Stage #2: with hydrogenchloride in 1,4-dioxane;tert-butyl methyl ether at 20; for 0.5 h;
Steps:
100.1 Step 1: 1-(Aminomethyl)cyclopentanol hydrochloride
To an ice cooled mixture of cyclopentanone (5.50 g, 65.0 mmol) and ZnBr2 (0.20 g, 8.00 mmol) was added TMSCN (10.0 mL, 73.4 mmol) and stirred at room temperature for 12 hours. The resulting cyanohydrin solution was added dropwise to a suspension of LiAlH4 (8.34 g, 219 mmol) in THF (30.0 mL) at 0° C. and mixture was refluxed for 1 hour. The reaction mixture was cooled to room temperature, water (10.0 mL), 4 M aqueous NaOH solution (10.0 mL) followed by water (10.0 mL) was added slowly to the reaction mixture with vigorous stirring. The resultant precipitate was filtered through a pad of celite, the organic phase separated and dried over KOH. The solution was decanted, dried (Na2SO4), filtered and concentrated. The residue was dissolved in MTBE (100 mL), 4N HCl in dioxane (10.0 mL) was added and mixture was stirred at room temperature for 30 minutes. The resulting precipitate was collected by filtering through sintered funnel to provide product (6.68 g, 68%) as a white solid.
References:
Merck Sharp & Dohme Corp.;Merck Canada Inc.;McCauley, John A.;Bennett, David Jonathan;Bungard, Christopher J.;Greshock, Thomas J.;Holloway, M. Katharine;Williams, Peter D.;Beaulieu, Christian;Crane, Sheldon;Lessard, Stephanie;Mckay, Daniel;Molinaro, Carmela;Moradei, Oscar Miguel;Sivalenka, Vijayasaradhi;Truong, Vouy Linh;Tummanapalli, Satyanarayana US2017/217986, 2017, A1 Location in patent:Paragraph 0564; 0565

120-92-3
427 suppliers
$11.00/25g
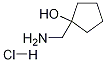
76066-27-8
45 suppliers
$44.00/250mg