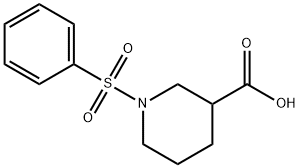
1-BENZENESULFONYL-PIPERIDINE-3-CARBOXYLIC ACID synthesis
- Product Name:1-BENZENESULFONYL-PIPERIDINE-3-CARBOXYLIC ACID
- CAS Number:321970-54-1
- Molecular formula:C12H15NO4S
- Molecular Weight:269.32
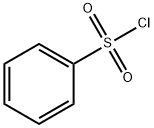
98-09-9
364 suppliers
$12.00/25g
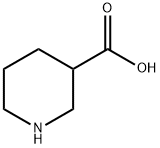
498-95-3
377 suppliers
$5.00/5g

321970-54-1
32 suppliers
$45.00/50mg
Yield:321970-54-1 97.83%
Reaction Conditions:
Stage #1: nipecotic acidwith sodium carbonate in water at 0; for 0.166667 h;
Stage #2: benzenesulfonyl chloride in water at 0 - 20;
Stage #3: with hydrogenchloride in water at 0 - 5; pH=3; for 0.25 - 0.5 h;
Steps:
1.1.A
Nipecotic acid (25 g, 193.79 mmol, 1 equiv.) was taken in a round bottomed flask. To this, sodium carbonate (61.62 g, 581.3 mmol, 3 equiv.) and water (250 mL)
References:
WO2008/87654,2008,A2 Location in patent:Page/Page column 24-25