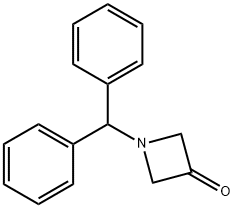
1-BENZHYDRYLAZETIDIN-3-ONE synthesis
- Product Name:1-BENZHYDRYLAZETIDIN-3-ONE
- CAS Number:40320-60-3
- Molecular formula:C16H15NO
- Molecular Weight:237.3
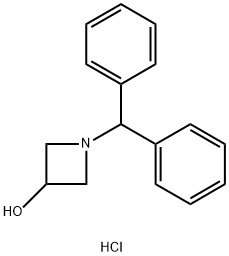
90604-02-7

40320-60-3
General procedure for the synthesis of 1-diphenylmethylazetidin-3-one: (Preparative Example 78) To a mixture of 1-diphenylmethylazetidin-3-ol hydrochloride (5.52 g) and triethylamine (27.9 ml) was added slowly and dropwise a dimethylformamide solution (80 ml) of pyridine trioxide complex (19.7 g). The reaction mixture was stirred at 50 °C for 30 min and then cooled to room temperature. The reaction mixture was poured into ice water, extracted with ethyl acetate and the organic layer was washed with brine. Activated carbon (5 g) was added to the organic layer and stirred at room temperature for 3 days. The activated carbon was removed by filtration and the filtrate was concentrated. The residue was dissolved in methanol (200 ml), activated carbon (10 g) was added and stirred at room temperature for 3 days. The activated carbon was removed by filtration again and the filtrate was concentrated. The residue was purified by silica gel column chromatography (eluent: heptane/ethyl acetate = 4:1, then 2:1). The grades containing the target compound were collected and concentrated to give a light yellow oil (3.21 g). Hexane was added to the oily material to induce crystallization and the crystals were collected by filtration. The crystals were dried under ventilated conditions to give the title compound (1.11 g, 23.4% yield). The concentrated residue of the filtrate was added to hexane and left to crystallize at room temperature. After precipitation, the supernatant was removed by pipette and dried under reduced pressure to give the light yellow crystalline title compound (940 mg, 19.8% yield).1H-NMR (CDCl3) δ (ppm): 4.01 (4H, s), 4.60 (1H, s), 7.22 (2H, m), 7.30 (4H, m), 7.48 (4H, m).
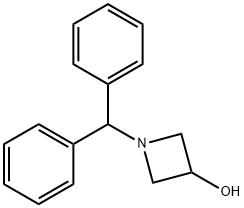
18621-17-5
384 suppliers
$5.00/1g

40320-60-3
231 suppliers
$6.00/1g
Yield:40320-60-3 96%
Reaction Conditions:
Stage #1:1-(diphenylmethyl)-3-hydroxyazetidine with oxalyl dichloride;dimethyl sulfoxide in dichloromethane at -78; for 1 h;
Stage #2: with triethylamine in dichloromethane
Steps:
1.a; 2.a Example 2:
a. Add oxalyl chloride (106 g, 0.84 mol) to a solution of dimethyl sulfoxide (65.2 g, 0.84 mol) in dichloromethane (2L).Control the reaction temperature at -78 ,Stir for 0.5 hours,Then, a 0.5 liter solution of compound 1 (100 g, 0.42 mol) in dichloromethane was added dropwise at the same temperature. The reaction solution was continuously stirred at -78 ° C for 1 hour.Triethylamine (422 g, 4.2 mol) was added to the reaction system.TLC (petroleum ether / ethyl acetate volume ratio = 1/1) showed the end of the reaction. The reaction solution was added dropwise to a saturated ammonium chloride (1.5 L) solution, and the organic phase was washed four times with 300 mL, dried over anhydrous sodium sulfate, and concentrated in vacuo to obtain a yellow solid compound 2 (96 g, yield 96%).
References:
Shanghai He Quan Pharmaceutical Co., Ltd.;An Ziqiang;Ren Wenwu;Zhou Qiang;Wang Ruiqi;Yang Fang;Yu Peidong;He Yanping;Liu Yueling;Liu Shengpan;Jiao Jiasheng;Xu Fujun;Zhang Lili;Chen Pei;Yu Lingbo;Ma Rujian CN110551133, 2019, A Location in patent:Paragraph 0006; 0013

90604-02-7
224 suppliers
$7.00/10g

40320-60-3
231 suppliers
$6.00/1g
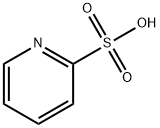
15103-48-7
119 suppliers
$7.00/250mg

18621-17-5
384 suppliers
$5.00/1g

40320-60-3
231 suppliers
$6.00/1g

79-37-8
490 suppliers
$17.67/10gm:

90604-02-7
224 suppliers
$7.00/10g

40320-60-3
231 suppliers
$6.00/1g
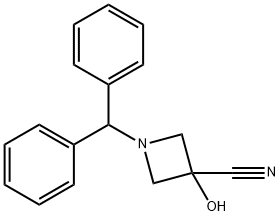
686347-59-1
4 suppliers
inquiry

40320-60-3
231 suppliers
$6.00/1g