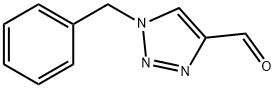
1-benzyl-1H-1,2,3-triazole-4-carbaldehyde synthesis
- Product Name:1-benzyl-1H-1,2,3-triazole-4-carbaldehyde
- CAS Number:124940-34-7
- Molecular formula:C10H9N3O
- Molecular Weight:187.2
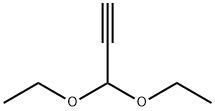
10160-87-9
204 suppliers
$8.00/1g

100-39-0
419 suppliers
$10.00/10 g

124940-34-7
20 suppliers
$110.00/50mg
Yield: 67%
Reaction Conditions:
with sodium azide;copper(II) sulfate;sodium L-ascorbate in water;tert-butyl alcohol at 70; for 24 h;
Steps:
S.3 General procedure for tandem substitution/deprotection/CuAAC.
General procedure: To a 20 mL screw-top vial was added (in order) CuSO4 (0.032 g, 0.2 mmol), L-ascorbic acid sodium salt (0.079 g, 0.4 mmol), NaN3 (0.065 g, 1.0 mmol), water (5 mL), t-butyl alcohol (5 mL), organic bromide reactant (1.0 mmol) and propargyl aldehyde diethyl acetal (0.158 mL, 1.1 mmol). The vial was then capped and stirred at either room temperature or 70 °C for 24 h. The reaction mixture was then extracted between CH2Cl2 and 5% NH4OH (aq). The organic layer was separated, dried with MgSO4 and gravity filtered. The solvent was removed via rotary evaporation to give the isolated product or product mixture used for NMR analysis. Further purification was accomplished by silica gel chromatography using either CH2CH2 or 20:1 CH2CH2:ethyl acetate eluent.
References:
Fletcher, James T.;Christensen, Joseph A.;Villa, Eric M. [Tetrahedron Letters,2017,vol. 58,# 47,p. 4450 - 4454] Location in patent:supporting information
28798-81-4
37 suppliers
$49.47/250mgs:

124940-34-7
20 suppliers
$110.00/50mg
14925-39-4
39 suppliers
$165.00/100mg
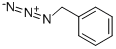
622-79-7
108 suppliers
$15.00/250mg

124940-34-7
20 suppliers
$110.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

124940-34-7
20 suppliers
$110.00/50mg

10160-87-9
204 suppliers
$8.00/1g

100-39-0
419 suppliers
$10.00/10 g
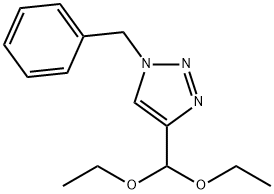
133735-81-6
7 suppliers
$301.00/1g

124940-34-7
20 suppliers
$110.00/50mg