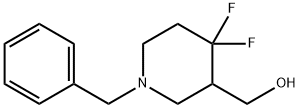
(1-Benzyl-4,4-difluoropiperidin-3-yl)methanol synthesis
- Product Name:(1-Benzyl-4,4-difluoropiperidin-3-yl)methanol
- CAS Number:1303973-25-2
- Molecular formula:C13H17F2NO
- Molecular Weight:241.28
1303974-56-2
34 suppliers
$120.00/1g

1303973-25-2
27 suppliers
$65.00/100mg
Yield:1303973-25-2 58.1 g
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 5;
Steps:
Intermediate 27: [4,4-Difluoro-1-(phenylmethyl)-3-piperidinyl]methanol
Intermediate 27: [4,4-Difluoro-1-(phenylmethyl)-3-piperidinyl]methanol
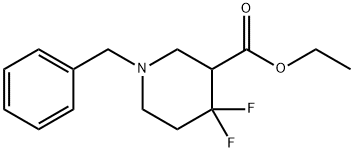
Ethyl 4,4-difluoro-1-(phenylmethyl)-3-piperidinecarboxylate (65.0 g, 0.230 mol) was dissolved in THF (900 ml).
The solution was cooled to 5° C., and lithium aluminium hydride (8.7 g, 0.230 mol, 1.0 eq) (Alfa) added in portions over 1 h, with the temperature kept below 5° C.
The mixture was removed from the cooling, and stirred for a further 90 min.
Once 1H NMR confirmed the absence of starting material, the reaction mixture was cooled to below 5° C., and ethyl acetate (325 ml) added (slightly exothermic), followed by saturated sodium potassium tartrate solution (solid NaK tartrate from Aldrich, 1 L) (exothermic, bubbles a lot).
The quenched mixture was allowed to reach room temperature and dichloromethane (1.5 L) added.
The mixture was stirred overnight then transferred to a separating funnel, and the layers separated.
The aqueous layer was extracted with dichloromethane (1.0 L), and the combined organic layers dried (MgSO4), and the solvent evaporated to give the product as a pale yellow oil (58.1 g).
GC 8.46 min.
References:
US2014/5188,2014,A1 Location in patent:Paragraph 0639; 0640; 0641
41276-30-6
100 suppliers
$61.96/1g

1303973-25-2
27 suppliers
$65.00/100mg