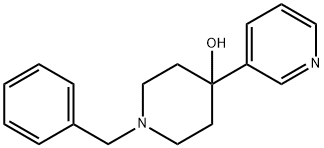
1-Benzyl-4-(Pyridin-3-Yl)Piperidin-4-Ol synthesis
- Product Name:1-Benzyl-4-(Pyridin-3-Yl)Piperidin-4-Ol
- CAS Number:188879-36-9
- Molecular formula:C17H20N2O
- Molecular Weight:268.35
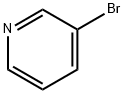
626-55-1
573 suppliers
$5.00/5/ G
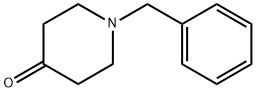
3612-20-2
446 suppliers
$6.00/10g

188879-36-9
11 suppliers
$459.00/250mg
Yield:188879-36-9 20%
Reaction Conditions:
Stage #1: 3-Bromopyridinewith n-butyllithium in tetrahydrofuran at -78; for 3 h;
Stage #2: 1-phenylmethyl-4-piperidone in tetrahydrofuran at -78; for 1.5 h;
Steps:
i
n-butyllithium (2 eq.) was added dropwise over a period of 2 hours at -78° C. to a solution of 3-bromopyridine (1 eq.) in dry tetrahydrofuran (250 ml). The reaction mixture was then stirred for 1 h at -78° C. N-benzyl-piperidone (3 g, in 50 ml of tetrahydrofuran) was slowly added dropwise to the solution over a period of 30 min. at -78° C. The reaction mixture was stirred for 1 h at -78° C., and the progress of the reaction was monitored by thin-layer chromatography. When the reaction was complete, water was added and extraction with ethyl acetate (3×100 ml) was carried out. The combined organic phases were dried (Na2SO4), filtered, and concentrated in vacuo. The crude product was purified by column chromatography (4% methanol in dichloromethane). Yield: 20%.
References:
US2008/153843,2008,A1 Location in patent:Page/Page column 76