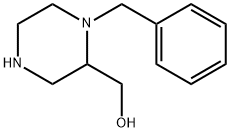
(1-Benzylpiperazin-2-yl)Methanol synthesis
- Product Name:(1-Benzylpiperazin-2-yl)Methanol
- CAS Number:476493-27-3
- Molecular formula:C12H18N2O
- Molecular Weight:206.28
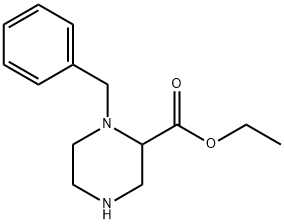
134749-45-4
5 suppliers
inquiry

476493-27-3
31 suppliers
$50.00/100mg
Yield:476493-27-3 70%
Reaction Conditions:
Stage #1: ethyl 1-benzylpiperazine-2-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Stage #2: with water;sodium hydroxide in tetrahydrofuran at 0;
Stage #3: with potassium carbonate in tetrahydrofuran;water at 20; for 0.333333 h;
Steps:
22.A
To a solution of 1-benzyl-piperazinee-2-carboxylic acid ethyl ester (synthesized as instructed in /) (500mg, 2.01mmol) in anhydrous tetrahydrofuran (10 mL) was slowly added lithium aluminum hydride (LiAlH4) (4 mL in 1.0 M THF, 4.02 mmol) at 0°C in a nitrogen atmosphere. The solution was stirred at room temperature for 2 hrs and then chilled to 0°C. Slow addition of water (100 μ?, 1N NaOH (100μ?), and water (300μ?) in the order produced a white slurry. To this was added a small amount of potassium carbonate, followed by stirring at room temperature for 20 min. After filtration through a cotton filter, the filtrate was purified using silica gel chromatography (eluent: dichloromethane/methanol, 10/1) and dried in a vacuum to afford 290mg of (1-benzyl-piperazin-2-yl)-methanol (white solid, yield 70%). 1H NMR (300MHz, CDCl3) δ 2.28(1H, m), 2.49(1H, m), 2.87(2H, m), 3.01(2H, m), 3.34(1H, d), 3.49-3.65(2H, d), 4.02(1H, dd), 4.07(1H, d), 7.25-7.32(5H, m))
References:
EP2390250,2011,A2 Location in patent:Page/Page column 82