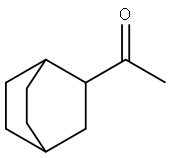
1-(Bicyclo[2.2.2]octan-2-yl)ethanone synthesis
- Product Name:1-(Bicyclo[2.2.2]octan-2-yl)ethanone
- CAS Number:23735-46-8
- Molecular formula:C10H16O
- Molecular Weight:152.23
![1-(Bicyclo[2.2.2]oct-5-en-2-yl)ethanone](/CAS/20150408/GIF/40590-77-0.gif)
40590-77-0
![1-(Bicyclo[2.2.2]octan-2-yl)ethanone](/CAS/20150408/GIF/23735-46-8.gif)
23735-46-8
General procedure for the synthesis of 1-(dicyclo[2.2.2]oct-5-en-2-yl)ethanone from 1-(dicyclo[2.2.2]oct-2-en-2-yl)ethanone: 1-(dicyclo[2.2.2]oct-5-en-2-yl)ethanone (1 g, 6.67 mmol) was dissolved in 50 mL of ethyl acetate and 10% palladium/carbon catalyst (57 mg) was added. The reaction mixture was stirred at room temperature using a Parr shaker under 20 psi hydrogen pressure for 5 hours. Upon completion of the reaction, the catalyst was removed by filtration through a diatomaceous earth pad, and the filtrate was concentrated under reduced pressure to afford the colorless oily product 1-(bicyclo[2.2.2]octan-2-yl)ethanone (1 g, 100% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 2.64 (m, 1H), 2.13 (s, 3H), 2.04 (m, 1H), 1.94 (m, 1H), 1.65 (m, 3H), 1.46-1.55 (m, 4H), 1.37-1.43 (m, 3H). No molecular ion peaks (MH+) were detected by mass spectrometry analysis.
![1-(Bicyclo[2.2.2]oct-5-en-2-yl)ethanone](/CAS/20150408/GIF/40590-77-0.gif)
40590-77-0
30 suppliers
$85.00/100mg
![1-(Bicyclo[2.2.2]octan-2-yl)ethanone](/CAS/20150408/GIF/23735-46-8.gif)
23735-46-8
26 suppliers
inquiry
Yield:23735-46-8 100%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen in ethyl acetate at 20; under 1034.32 Torr; for 5 h;
Steps:
2.53c 1-(bicyclo[2.2.2]octan-2-yl)ethanone
A solution of 1-(bicyclo[2.2.2]oct-5-en-2-yl)ethanone (Example 2.51c) (1 g, 6.67 mmol) in 50 mL of EtOAc was hydrogenated on 10% Pd/C (57 mg) in Parr shaker at 20 psi at room temperature for 5 h. Then reaction mixture was filtered trough Celite pad and the filtrate was concentrated in vacuum to obtain 90% clean ketone as a colorless oil (1 g, 100%). 1H NMR (400 MHz, CDCl3) δ 2.64 (m, 1H), 2.13 (s, 3H), 2.04 (m, 1H), 1.94 (m, 1H), 1.65 (m, 3H), 1.46-1.55 (m, 4H), 1.37-1.43 (m, 3H). MS N/A (MH+).
References:
US2015/376136,2015,A1 Location in patent:Paragraph 0583
1165952-91-9
1 suppliers
inquiry
![1-(Bicyclo[2.2.2]octan-2-yl)ethanone](/CAS/20150408/GIF/23735-46-8.gif)
23735-46-8
26 suppliers
inquiry
![Bicyclo[2.2.2]octane-2-carbonyl chloride (9CI)](/CAS/GIF/68569-32-4.gif)
68569-32-4
1 suppliers
inquiry
![1-(Bicyclo[2.2.2]octan-2-yl)ethanone](/CAS/20150408/GIF/23735-46-8.gif)
23735-46-8
26 suppliers
inquiry