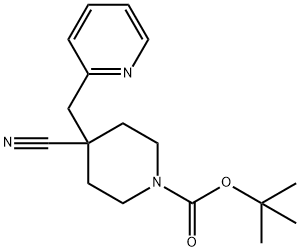
1-BOC-4-CYANO-4-(2-PYRIDINYLMETHYL)-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-(2-PYRIDINYLMETHYL)-PIPERIDINE
- CAS Number:895132-38-4
- Molecular formula:C17H23N3O2
- Molecular Weight:301.38
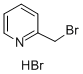
31106-82-8
185 suppliers
$10.00/1g
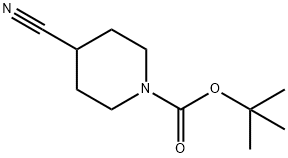
91419-52-2
362 suppliers
$6.00/1g

895132-38-4
4 suppliers
inquiry
Yield:895132-38-4 48.84 %
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;trifluoroacetic acid
Steps:
37.1 Tert-butyl 4-cyano-4-(pyridin-2-ylmethyl)piperidine-1-carboxylate
Step 1
Tert-butyl 4-cyano-4-(pyridin-2-ylmethyl)piperidine-1-carboxylate
2-(bromomethyl)pyridine hydrobromide 37a (2.65 g, 10.46 mmol), tert-butyl 4-cyanopiperidine-1-carboxylate (2 g, 9.51 mmol) and N,N-diisopropylethylamine (1.60 g, 12.36 mmol) were added to 2 mL of toluene, stirred at room temperature for 15 minutes, cooled to 0°C, dropwise added with a 1 M tetrahydrofuran solution (2.09 g, 10.46 mmol) containing potassium bis(trimethylsilyl)amide, then heated to room temperature, and reacted for 1 hour.
After the reaction was completed, the reaction solution was added with 20 mL of saturated sodium chloride solution and extracted with ethyl acetate (20 mL*3), and then washed with a saturated sodium chloride solution (20 mL).
Organic phases were dried with anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and subjected to liquid phase separation (separation column: AKZONOBEL Kromasil; 250*21.2 mm I.D.; 5 μm, 20 mL/min; mobile phase A: 0.05% TFA + H2O, and mobile phase B: CH3CN) to obtain tert-butyl 4-cyano-4-(pyridin-2-ylmethyl)piperidine-1-carboxylate 37b (1.4 g) with a yield of 48.84%.
MS m/z (ESI): 302.0 [M+1]
References:
EP4092019,2022,A1
4377-33-7
105 suppliers
$90.00/250mg

91419-52-2
362 suppliers
$6.00/1g

895132-38-4
4 suppliers
inquiry