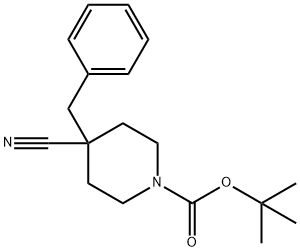
1-BOC-4-CYANO-4-BENZYL-PIPERIDINE synthesis
- Product Name:1-BOC-4-CYANO-4-BENZYL-PIPERIDINE
- CAS Number:906329-30-4
- Molecular formula:C18H24N2O2
- Molecular Weight:300.4

100-39-0
421 suppliers
$10.00/10g
91419-52-2
361 suppliers
$6.00/1g

906329-30-4
17 suppliers
inquiry
Yield:906329-30-4 70.4%
Reaction Conditions:
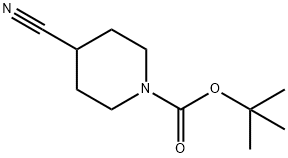
Stage #1: 1-tert-butoxycarbonyl-4-cyanopiperidinewith n-butyllithium;diisopropylamine in tetrahydrofuran;hexane at -70 - 0; for 0.5 h;
Stage #2: benzyl bromide in tetrahydrofuran;hexane at 75; for 5 h;
Steps:
15 Synthesis of A29
To a solution of DIPA (5.75 g, 56.9 mmol) in THF (40 mL) was added BuLi (22.7 mL, 2.5 M in hexane, 56.9 mmol) at -70 °C. The mixture was warmed to 0 °C and stirred for 30 min to give an LDA solution. To the cold (0°C) LDA solution was added a solution of tert-butyl 4-cyanopiperidine-1-carboxylate (10 g, 47.5 mmol) in THF (20 mL). After stirring at 0 °C for 0.5 h, (bromomethyl)benzene (9.73 g, 56.9 mmol) was added. The mixture was stirred at 75 °C for 5 h, poured into water (100 mL) and extracted with EtOAc (3 x 100 mL). The combined organic phase was washed with brine (2 x 100 mL), dried over Na2SO4 and concentrated. The residue was purified by flash chromatography using a gradient elution (0~30% EtOAc in PE) to give tert-butyl 4-benzyl-4-cyanopiperidine-1-carboxylate (10g, 70.4% yield) as a solid and used directly for the next step.
References:
WO2020/243027,2020,A1 Location in patent:Paragraph 0174

100-39-0
421 suppliers
$10.00/10g

906329-30-4
17 suppliers
inquiry