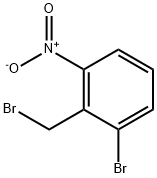
1-broMo-2-(broMoMethyl)-3-nitrobenzene synthesis
- Product Name:1-broMo-2-(broMoMethyl)-3-nitrobenzene
- CAS Number:58579-54-7
- Molecular formula:C7H5Br2NO2
- Molecular Weight:294.93
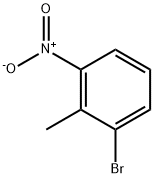
55289-35-5

58579-54-7
General procedure for the synthesis of 2-bromo-6-nitrobenzyl bromide from 2-bromo-6-nitrotoluene: Example 5: Synthesis of 12-amino-6-bromobenzyl alcohol (Intermediate I-1) 1. 2-Nitro-6-bromotoluene (32.20 g, 0.20 mol), N-bromosuccinimide (26.6 g, 0.20 mol) and benzoyl peroxide (0.40 g, 2 mmol) were dissolved in chlorobenzene (100 mL) and heated to reflux for about 20 hours. After completion of the reaction, the reaction mixture was filtered and the filtrate was collected. The filtrate was concentrated under vacuum to give a light brown oily product. Recrystallization by ethanol afforded 2-nitro-6-bromobenzyl bromide (47.31 g, 81% yield) as a yellow solid. 2. 2-Nitro-6-bromobenzyl bromide (14.70 g, 0.05 mol) and anhydrous sodium acetate (14.7 g, 0.15 mol) were added to N,N-dimethylformamide (70 mL) and reacted by heating to 70 °C for about 1 h, followed by cooling to room temperature. The reaction mixture was poured into water (150 mL) and extracted three times with ethyl acetate. The organic phases were combined, washed three times sequentially with water and saturated sodium chloride solution, dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under vacuum to give methyl 2-nitro-6-bromophenylacetate (12.65 g, 92% yield) as a white solid. 3. 2-Nitro-6-bromophenylacetic acid methyl ester (2.73 g, 0.01 mol) was added to water (100 mL) and 10% potassium hydroxide solution (20 mL) was added dropwise. The reaction mixture was heated to reflux for 2 h. After cooling to room temperature, it was extracted three times with ethyl acetate. The organic phases were combined, washed sequentially with water, saturated sodium chloride solution, dried with anhydrous sodium sulfate and filtered. The filtrate was concentrated under vacuum to give 2-bromo-6-nitrobenzenemethanol (1.91 g, 83% yield) as a yellow solid. 4. 2-Bromo-6-nitrobenzenemethanol (1.15 g, 5 mmol), sodium sulfide (0.78 g, 10 mmol), and sulfur (0.32 g, 10 mmol) were dissolved in a solvent mixture of ethanol (50 mL) and water (25 mL), heated to reflux for 2 hours, and subsequently cooled to room temperature. The ethanol was removed by vacuum concentration and the remaining reaction mixture was added to water (30 mL) and extracted three times with ether. The organic phases were combined, washed sequentially with water, saturated sodium chloride solution, dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under vacuum to give 2-bromo-6-aminobenzyl alcohol (0.78 g, 78% yield) as a yellow solid, [M + H]+ = 202.

55289-35-5
306 suppliers
$13.00/1g

58579-54-7
75 suppliers
$17.00/100mg
Yield: 100%
Reaction Conditions:
with N-Bromosuccinimide;Perbenzoic acid in tetrachloromethane for 192 h;Heating;Irradiation;
References:
Hewson, Alan T.;Hughes, Kevin;Richardson, Steward K.;Sharpe, David A.;Wadsworth, Alan H. [Journal of the Chemical Society. Perkin transactions I,1991,# 6,p. 1565 - 1569]
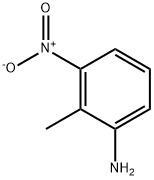
603-83-8
310 suppliers
$11.00/5g

58579-54-7
75 suppliers
$17.00/100mg