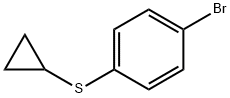
1-BroMo-4-cyclopropylthiobenzene synthesis
- Product Name:1-BroMo-4-cyclopropylthiobenzene
- CAS Number:411229-63-5
- Molecular formula:C9H9BrS
- Molecular Weight:229.14
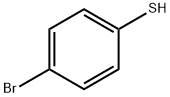
106-53-6
286 suppliers
$12.00/1g
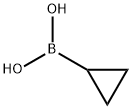
411235-57-9
427 suppliers
$6.00/1g

411229-63-5
14 suppliers
$65.00/100mg
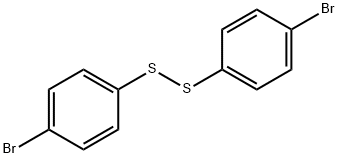
5335-84-2
72 suppliers
$45.00/100mg
Yield:411229-63-5 95% ,5335-84-2 2%
Reaction Conditions:
with [2,2]bipyridinyl;copper diacetate;caesium carbonate in 1,2-dichloro-ethane at 70; for 16 h;Sealed tube;
Steps:
General procedure for the synthesis of arylcyclopropyl sulfides
General procedure: A sealed tube equipped with a magnetic stirring bar wascharged under ambiant air with cyclopropylboronic acid (25,0.6 mmol, 1.5 equiv), cesium carbonate (0.4 mmol, 1.0 equiv),Cu(OAc)2 (0.4 mmol, 1.0 equiv), 2,2'-bipyridine (0.4 mmol,1.0 equiv) and thiophenol 14 (0.4 mmol, 1.0 equiv). Dichloroethane(0.1 M) was added, the tube was sealed and heated at70 °C for 16 hours. The reaction mixture was cooled to roomtemperature and aqueous NH4OH 25% (5 mL) was added. Thereaction mixture was stirred for a few minutes, transferred in aseparatory funnel and extracted with DCM (3 × 5 mL). Thecombined organic layers were washed with brine (2 × 10 mL),dried over anhydrous Na2SO4 and concentrated under reduced pressure. The residue was purified by flash column chromatographyusing the indicated solvent system to afford the correspondingaryl cyclopropyl sulfide 1 and diaryl disulfide 26 as aside-product
References:
Benoit, Emeline;Fnaiche, Ahmed;Gagnon, Alexandre [Beilstein Journal of Organic Chemistry,2019,vol. 15,p. 1162 - 1171]
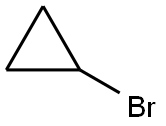
4333-56-6
393 suppliers
$6.00/5g

106-53-6
286 suppliers
$12.00/1g

411229-63-5
14 suppliers
$65.00/100mg

4333-56-6
393 suppliers
$6.00/5g

411229-63-5
14 suppliers
$65.00/100mg
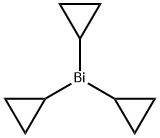
925430-09-7
0 suppliers
inquiry

106-53-6
286 suppliers
$12.00/1g

411229-63-5
14 suppliers
$65.00/100mg

5335-84-2
72 suppliers
$45.00/100mg
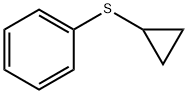
14633-54-6
146 suppliers
$22.00/1g

411229-63-5
14 suppliers
$65.00/100mg