
1-Bromo-4-(neopentyloxy)benzene synthesis
- Product Name:1-Bromo-4-(neopentyloxy)benzene
- CAS Number:528528-58-7
- Molecular formula:C11H15BrO
- Molecular Weight:243.14
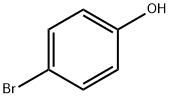
106-41-2
503 suppliers
$13.00/5g
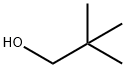
75-84-3
271 suppliers
$10.00/1g

528528-58-7
14 suppliers
$45.00/100mg
Yield:528528-58-7 75%
Reaction Conditions:
Stage #1: 2,2-dimethyl-propanol-1with methanesulfonyl chloride;triethylamine in N,N-dimethyl-formamide at 0; for 0.5 h;
Stage #2: 4-bromo-phenolwith tetra-(n-butyl)ammonium iodide;caesium carbonate in N,N-dimethyl-formamide at 80 - 130; for 18.25 h;Inert atmosphere;
Steps:
I22 Example I22: 1 -Bromo-4-(2,2-dimethylpropoxy)benzene
Neopentyl alcohol (2, 2-dimethyl-1 -propanol, 2.93 ml, 27 mmol) was dissolved in 50 ml of dry L/,L -dimethylformamide. The solution was cooled to 0°C and triethylamine (3.78 ml, 27 mmol) was added while stirring, followed by slow, dropwise addition of methanesulfonyl chloride (1 .94 ml, 25 mmol). After formation of triethylamine hydrochloride precipitate, the contents of the reaction vessel were stirred for further 30 minutes without cooling, and subsequently commercially available 4-bromophenol (3.53 g, 20 mmol, CAS [106-41 -2] ), tetrabutylammonium iodide (0.923 g, 2.5 mmol) and cesium carbonate (18.5 g, 56.2 mmol) were added and the reaction mixture was heated to 80° C with simultaneous blowing with argon for 15 minutes. Then temperature of the reaction mixture was raised to 130°C and stirring was continued for 18 hours. After cooling, reaction product was isolated by the addition of water (150 ml) and extraction with diethyl ether. Organic phase was separated, washed with water (2x 50 ml) and brine (50 ml), dried, concentrated and purified by chromatography (silica gel 60, 230 - 400 mesh, eluent: heptane- ethyl acetate gradient from 20: 1 to 8: 1 ). The title compound was obtained as a colorless oil (3.66 g, 75% yield).1H NMR (300 MHz, CDCl3) 5: 7.37 - 7.30 (m, 2H), 6.79 - 6.72 (m, 2H), 3.53 (s, 2H), 1.01 (s, 9H).13C NMR (75 MHz, CDCI3) d: 158.86, 132.26, 116.46, 112.58, 78.27, 32.03, 26.71.
References:
WO2019/134984,2019,A1 Location in patent:Page/Page column 48; 49