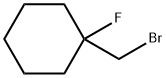
1-(bromomethyl)-1-fluorocyclohexane synthesis
- Product Name:1-(bromomethyl)-1-fluorocyclohexane
- CAS Number:17171-00-5
- Molecular formula:C7H12BrF
- Molecular Weight:195.07
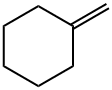
1192-37-6
84 suppliers
$97.29/1g

17171-00-5
6 suppliers
inquiry
Yield:17171-00-5 73%
Reaction Conditions:
with N-Bromosuccinimide;triethylamine tris(hydrogen fluoride) in dichloromethane at 5 - 20;
Steps:
β-Fluoro Bromides 2a-t and 2v-x; General Procedure
General procedure: Et3N·3HF (20.2 g, 125 mmol) was added to a solution of alkene 1a-l,n-t,v-x (50 mmol) in CH2Cl2 (100 mL), and the resulting mixture was cooled to 5 °C. Then, NBS (10.7 g, 60 mmol) was added portionwise at 5 °C, and the reaction mixture was stirred at rt overnight. Then, it was poured onto an ice-water mixture (200 g). The organic layer was separated, washed with saturated aq NaHCO3 (3 × 50 mL), dried (Na2SO4), and evaporated under reduced pressure, affording pure product (2a,g,i,o,p,r-t), or crude product which was purified by distillation (2e,h,j,q,w,x), column chromatography (2c,l-n,v), recrystallization (2d,f), or used without purification (2b,k).
References:
Gurbanov, Rustam;Sokolov, Andriy;Golovach, Sergey;Melnykov, Kostiantyn;Dobrydnev, Alexey V.;Grygorenko, Oleksandr O. [Synthesis,2021,vol. 53,# 10,art. no. SS-2020-T0036-OP,p. 1771 - 1784]
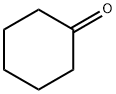
108-94-1
584 suppliers
$12.00/50g

17171-00-5
6 suppliers
inquiry