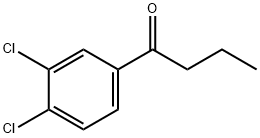
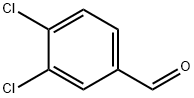
1-Butanone, 1-(3,4-dichlorophenyl)- synthesis
- Product Name:1-Butanone, 1-(3,4-dichlorophenyl)-
- CAS Number:6582-45-2
- Molecular formula:C10H10Cl2O
- Molecular Weight:217.09
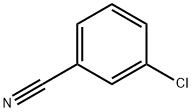
766-84-7
271 suppliers
$9.00/10g
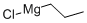
2234-82-4
109 suppliers
$63.95/100ml

6582-45-2
11 suppliers
$45.00/25mg
Yield:6582-45-2 83%
Reaction Conditions:
Stage #1: 3-chloro-benzonitrile;1-propylmagnesium chloride in tetrahydrofuran at 0 - 20; for 144 h;Inert atmosphere;
Stage #2: with hydrogenchloride in tetrahydrofuran;water at 0;
Stage #3: with sodium hydrogencarbonate in tetrahydrofuran;water;
Steps:
1.a.1
Synthesis of 2-(te^-Butylamino)-3',4'-dichlorobutyrophenone (2aa) Step 1. 3',4'-Dichlorobutyrophenone (9aa). To a solution of 3.00 g (0.017 mol) of 8e in 40 mL of dry tetrahydrofuran cooled to 0 0C was added drop wise 21 mL (2.0 M in Et2O) of propylmagnesium chloride. The reaction solution was allowed to warm to room temperature and stirred at room temperature for 144 h under nitrogen. The reaction solution was cooled to 0 °C and 150 mL of a 5% aqueous hydrochloric acid solution was added drop wise. After stirring overnight at room temperature, the reaction mixture was quenched with a saturated aqueous sodium bicarbonate solution, and the product was extracted with methylene chloride, dried (Na2SO4), and filtered. The solvent was removed, and the resulting residue was dried briefly under high vacuum to give 3.87 g of a brown oil. Purification by flash chromatography (silica, 5:1 hexane-methylene chloride) gave 3.15 g (83%) of 9aa as a yellow oil. 1H NMR(CDCl3) δ 8.03 (s, IH), 7.80-7.76 (dd, IH), 7.54 (d, IH), 2.91 (t, 2H), 1.84-1.69 (m, 2H), 1.00 (t, 3H).
References:
WO2010/121022,2010,A1 Location in patent:Page/Page column 60
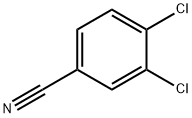
6574-99-8
358 suppliers
$10.00/1g
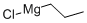
2234-82-4
109 suppliers
$63.95/100ml

6582-45-2
11 suppliers
$45.00/25mg
557-93-7
226 suppliers
$45.10/5g
6287-38-3
259 suppliers
$8.00/10g

6582-45-2
11 suppliers
$45.00/25mg