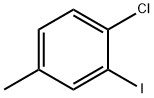
1-chloro-2-iodo-4-methyl-benzene synthesis
- Product Name:1-chloro-2-iodo-4-methyl-benzene
- CAS Number:2401-22-1
- Molecular formula:C7H6ClI
- Molecular Weight:252.48
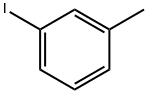
625-95-6
254 suppliers
$14.00/25g

2401-22-1
27 suppliers
inquiry
Yield:2401-22-1 92.1%
Reaction Conditions:
with sulfuryl dichloride;diphenyl sulfide;antimony(III) chloride in 1,2-dichloro-ethane at 20 - 25; for 6 h;
Steps:
12 [Example 12]
1 g of m-iodotoluene (4.59 mmol; manufactured by Manac),0.021 g (0.09 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of antimony chloride (III) and 0.017 g (0.09 mmol, diphenyl sulfide manufactured by Wako Pure Chemical Industries, Ltd.) were dissolved in 5 g of ethylene dichloride,Subsequently, 0.74 g (5.5 mmol; manufactured by Wako Pure Chemical Industries, Ltd.) of sulfuryl chloride was added,The reaction was carried out at 20 to 25 ° C. for 6 hours. After completion of the reaction,A 10 wt% hydrochloric acid aqueous solution was added,After stirring for a while,The organic layer was separated.Then,Neutralized with a 10 wt% aqueous sodium hydroxide solution,The organic layer was separated.The purity of the target product and the by-product content in the obtained reaction solution were measured by GC.Monochloro-3-iodotoluene: 92.10%, halosubstituted product (by-productThing): 0.10%.
References:
JP6074279,2017,B2 Location in patent:Paragraph 0048
202982-72-7
71 suppliers
$15.00/100mg

74-88-4
356 suppliers
$15.00/10g

2401-22-1
27 suppliers
inquiry
95-81-8
186 suppliers
$10.00/1g

2401-22-1
27 suppliers
inquiry
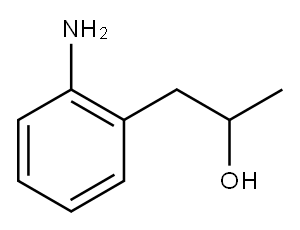
65826-91-7
2 suppliers
inquiry

2401-22-1
27 suppliers
inquiry