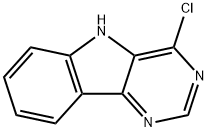
1-CHLORO-9H-2,4,9-TRIAZA-FLUORENE synthesis
- Product Name:1-CHLORO-9H-2,4,9-TRIAZA-FLUORENE
- CAS Number:98792-02-0
- Molecular formula:C10H6ClN3
- Molecular Weight:203.63
![4H-Pyrimido[5,4-b]indol-4-one, 3,5-dihydro-](/CAS/GIF/61553-71-7.gif)
61553-71-7
28 suppliers
$43.00/100mg

98792-02-0
19 suppliers
$45.00/5mg
Yield: 72.06%
Reaction Conditions:
with trichlorophosphate for 2 h;Reflux;
Steps:
1
5H-pyrimido[5,4-b]indol-4-one (0.62g, 3.33mmol) dissolved in 3ml of phosphorus oxychloride was heated to reflux for about 2h. Unreacted phosphorus oxychloride was distilled off. The residue was added to ice water. Saturated aqueous sodium carbonate adjusted pH to about 10. It was extracted with ethyl acetate thre times. The combined organic phases were successively washed with water and brine. Dried over anhydrous sodium sulfate. It was filtered and the liquid portion was concentrated under reduced pressure giving a crude pale yellow solid. Column chromatography gave the intermediate 4-chloro-5H-pyrimido[5,4-b]indole (M-1) 0.49g, yield 72.06%.
References:
Shanghai Pharmaceutical Industry Research Academy;Li, Jiangqi;Zhang, Qingwei;Du, Zhenxin;Lu, Xiulian;Zhang, Baoyin;Zhang, Li CN104045642, 2016, B Location in patent:Paragraph 0094
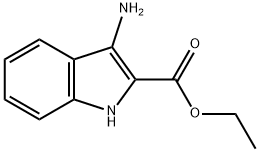
87223-77-6
73 suppliers
$87.00/100mg

98792-02-0
19 suppliers
$45.00/5mg
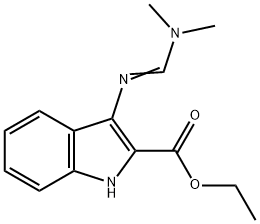
97311-03-0
1 suppliers
inquiry

98792-02-0
19 suppliers
$45.00/5mg
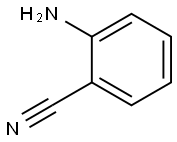
1885-29-6
452 suppliers
$5.00/1g

98792-02-0
19 suppliers
$45.00/5mg
25116-00-1
24 suppliers
inquiry

98792-02-0
19 suppliers
$45.00/5mg