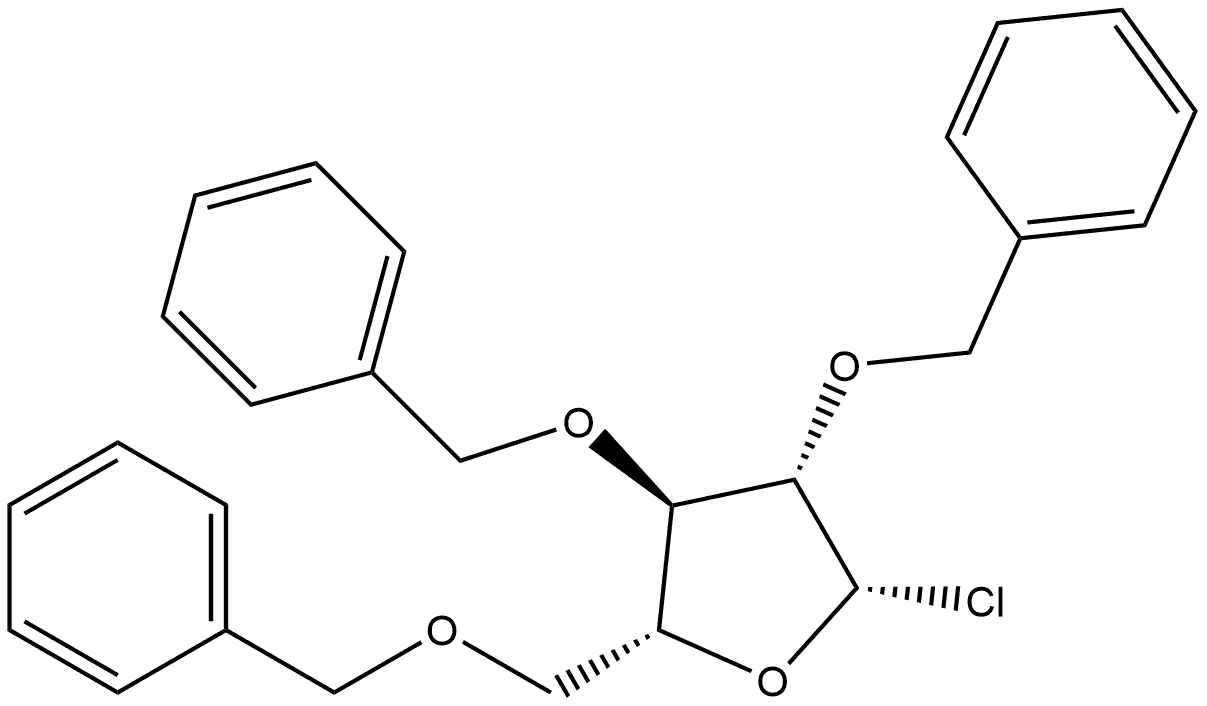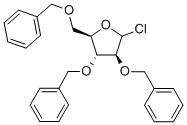
1-chloro-Tri-2,3,5-O-benzyl-D-arabofuranose synthesis
- Product Name:1-chloro-Tri-2,3,5-O-benzyl-D-arabofuranose
- CAS Number:4060-34-8
- Molecular formula:C26H27ClO4
- Molecular Weight:438.94
Yield:66.667 % de
Reaction Conditions:
with pyridine;bis(trichloromethyl) carbonate in tetrahydrofuran at 0 - 20; for 1 h;Inert atmosphere;
Steps:
4.1. Method A (triphosgene/pyridine) [48]
General procedure: Triphosgene (1 mmol, 0.5 eq.) was added to a solution of substrate 1(2 mmol, 1 eq.) in anhydrous THF (10 mL) under Ar atmosphere. The solution was cooled to 0 °C and pyridine (3.2 mmol, 1.6 eq.) was added dropwise. The formation of a white precipitate (pyridinium chloride) was observed immediately during the addition. The reaction was stirred at room temperature for 1 h and then filtered. The filtrate was concentrated under reduced pressure, dissolved in EtOAc (100 mL) and washed with H2O (2 × 100 mL). The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The resultingoil was kept under high vacuum for 1 h.
References:
Choutka, Jan;Kratochvíl, Michal;Parkan, Kamil;Pohl, Radek;Zyka, Jakub [Carbohydrate Research,2020,vol. 496] Location in patent:supporting information
52522-49-3
155 suppliers
$11.00/250mg

4060-34-8
13 suppliers
inquiry
62255-44-1
1 suppliers
inquiry

4060-34-8
13 suppliers
inquiry

52522-49-3
155 suppliers
$11.00/250mg

4060-34-8
13 suppliers
inquiry
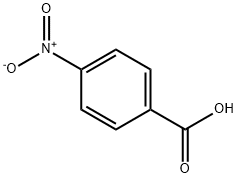
62-23-7
660 suppliers
$14.00/25g
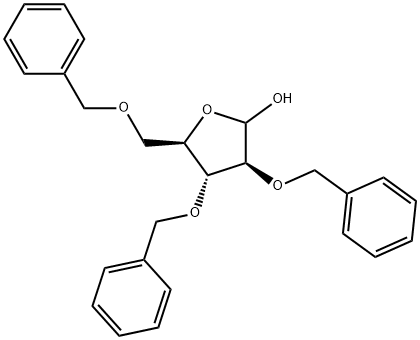
160549-10-0
18 suppliers
inquiry

4060-34-8
13 suppliers
inquiry