
(1-cyclohexylpyrrolidin-3-yl)methanol synthesis
- Product Name:(1-cyclohexylpyrrolidin-3-yl)methanol
- CAS Number:100049-71-6
- Molecular formula:C11H21NO
- Molecular Weight:183.29

93102-02-4
0 suppliers
inquiry

100049-71-6
15 suppliers
$31.00/250mg
Yield:100049-71-6 92.3%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at -10; for 0.5 h;
Steps:
132.B Step B: (1-cyclohexylpyrrolidin-3-yl)methanol:
To a solution of methyl 1- cyclohexylpyrrolidine-3-carboxylate (1.00 g, 4.73 mmol) in THF (20 mL) was added LiAlH4 (413 mg, 10.9 mmol) at -10 °C. The reaction mixture was stirred at -10 °C for 0.5 hour. The reaction mixture was quenched by addition of saturated Na2S04 (2 mL) and mixture filtered and the filter cake washed with THF (3 χ 50 mL).The combined organics were concentrated under vacuum to give : (l-cyclohexylpyrrolidin-3-yl)methanol (800 mg, 4.36 mmol, 92.3 % yield) as a coloress oil. MR (400MHz, CHLOROFORM-d) δ = 3.71 (dd, J= 4.1, 10.0 Hz, 1H), 3.53 (dd, J=4.4, 10.0 Hz, 1H), 2.90 (dt, J=4.4, 8.8 Hz, 1H), 2.74 (dd, J= 3.2, 8.8 Hz, 1H), 2.53 (dd, J= 6.8, 8.8 Hz, 1H), 2.36 - 2.24 (m, 2H), 2.04 - 1.93 (m, 2H), 1.90 (br s, 2H), 1.78 - 1.64 (m, 3H), 1.61 - 1.52 (m, 1H), 1.33 - 1.12 (m, 5H)
References:
WO2017/201161,2017,A1 Location in patent:Paragraph 0534
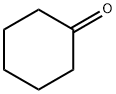
108-94-1
583 suppliers
$12.00/50g

100049-71-6
15 suppliers
$31.00/250mg
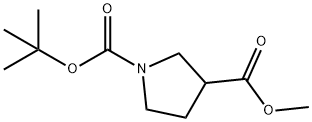
122684-33-7
234 suppliers
$8.00/1g

100049-71-6
15 suppliers
$31.00/250mg