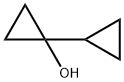
1-cyclopropylcyclopropan-1-ol synthesis
- Product Name:1-cyclopropylcyclopropan-1-ol
- CAS Number:54251-80-8
- Molecular formula:C6H10O
- Molecular Weight:98.14
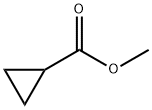
2868-37-3
283 suppliers
$10.00/25g
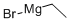
925-90-6
363 suppliers
$12.00/5ml

54251-80-8
20 suppliers
$398.00/1g
Yield:54251-80-8 91%
Reaction Conditions:
with titanium isopropoxide in 2-methyltetrahydrofuran at 15 - 20; for 4 h;Solvent;Temperature;
Steps:
1.1; 41 Step 1: Synthesis of [1,1′-bi(cyclopropan)]-1-ol (18)
A solution of methyl cyclopropanecarboxylate (109 g, 1.09 mol) in 2-MeTHF (1.31 L) and titanium(IV) isopropoxide (71 mL, 240.6 mmol) was stirred in a Morton flask then cooled to 18° C. Ethylmagnesium bromide (753 mL of 3 M, 2.259 mol) was added dropwise over 2 h to control the temperature between 15 to 20° C. The mixture was stirred for another 2 h then cooled to 5° C. and quenched drop-wise (slowly) with cold (5-10° C.) NaHSO4 (1.31 L of 20% w/v, 2.182 mol) while keeping the temperature below 10° C. The organic phase was isolated and the aqueous phase was re-extracted with hexanes (500 mL). The aqueous phase was discarded. The organic phases were combined, washed with sat. aq. NaHCO3(200 mL of 10% w/v, 238 mmol), dried over Na2SO4, and concentrated (30° C./40 torr) to afford 108.6 g of [1,1′-bi(cyclopropan)]-1-ol as a pale yellow liquid. The sample contained 8 wt % 2-MeTHF and 2 wt % iPrOH by 1H NMR, so the corrected yield of the desired product was 91%.1H NMR (400 MHz, Chloroform-d) δ 1.99 (s, 1H), 1.35 (tt, J=8.2, 5.1 Hz, 1H), 1.22 (dd, J=9.0, 6.1 Hz, 1H), 0.70-0.65 (m, 2H), 0.52-0.45 (m, 2H), 0.43-0.38 (m, 2H), 0.21-0.15 (m, 2H).
References:
US2021/47345,2021,A1 Location in patent:Paragraph 0418-0420; 0756; 0757
16492-07-2
1 suppliers
inquiry

54251-80-8
20 suppliers
$398.00/1g
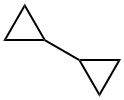
5685-46-1
1 suppliers
inquiry

54251-80-8
20 suppliers
$398.00/1g
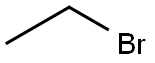
74-96-4
458 suppliers
$9.00/5g

2868-37-3
283 suppliers
$10.00/25g

54251-80-8
20 suppliers
$398.00/1g
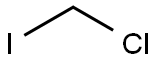
593-71-5
311 suppliers
$6.00/5g
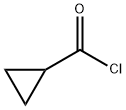
4023-34-1
367 suppliers
$12.00/5g

54251-80-8
20 suppliers
$398.00/1g