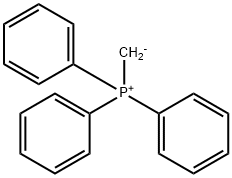
1-Dodecene,12-broMo- synthesis
- Product Name:1-Dodecene,12-broMo-
- CAS Number:99828-63-4
- Molecular formula:C12H23Br
- Molecular Weight:247.21

3344-70-5

99828-63-4
The general procedure for the synthesis of 12-bromo-1-dodecatriene from 1,12-dibromododecane is as follows: 1,12-dibromodecane (1 eq.) was dissolved in anhydrous THF (0.1 M) under argon protection with stirring. Potassium tert-butoxide (1.15 eq.) was added in batches over 30 min. The reaction mixture was heated to reflux and stirred for 16 hours. After completion of the reaction, it was cooled to room temperature and the reaction was quenched with water. The reaction mixture was diluted with diethyl ether and the organic and aqueous layers were separated. The aqueous layer was extracted several times with diethyl ether, all organic layers were combined, washed with saturated brine and dried over anhydrous sodium sulfate. After filtration, the filtrate was concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel fast column chromatography using petroleum ether as eluent to obtain the target product 12-bromo-1-dodecene.

3344-70-5
216 suppliers
$5.00/1g

99828-63-4
54 suppliers
$108.00/250mg
Yield:99828-63-4 66%
Reaction Conditions:
with potassium tert-butylate in tetrahydrofuran for 16 h;Inert atmosphere;Reflux;
Steps:
2.3 General Procedure for the synthesis of 1-bromo-ω-alkenes
General procedure: To a stirred solution of n-dibromoalkane (1 equiv.) in anhydrous THF (0.1M) under an argon atmosphere was added tert-BuOK (1.15 equiv.) in portionwise over 30 min. After being stirred under reflux for 16h, the reaction was cooled and subsequently quenched with water. The resulting mixture was then diluted with diethylether, and the layers were separated. The aqueous layer was extracted several times with diethylether, and the combined organic layers were washed with brine, dried over anhydrous Na2SO4, filtered and concentrated under reduced pressure. The resultant crude product was purified by flash column chromatography over silica gel using petroleum ether as eluent to afford the desired product.
References:
Saied, Essa M.;Le, Thuy Linh-Stella;Hornemann;Arenz, Christoph [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 14,p. 4047 - 4057] Location in patent:supporting information

35289-31-7
13 suppliers
inquiry

99828-63-4
54 suppliers
$108.00/250mg

1611-56-9
251 suppliers
$8.00/5g

18107-18-1
210 suppliers
$33.00/5g

99828-63-4
54 suppliers
$108.00/250mg
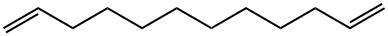
5876-87-9
44 suppliers
$55.00/1 mL

3344-70-5
216 suppliers
$5.00/1g

99828-63-4
54 suppliers
$108.00/250mg