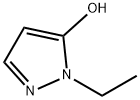
1-ethyl-1H-pyrazol-5-ol synthesis
- Product Name:1-ethyl-1H-pyrazol-5-ol
- CAS Number:107296-34-4
- Molecular formula:C5H8N2O
- Molecular Weight:112.13
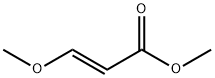
5788-17-0
171 suppliers
$21.00/5mL
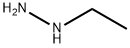
624-80-6
36 suppliers
inquiry

107296-34-4
16 suppliers
$108.00/100mg
Yield:-
Reaction Conditions:
Stage #1: methyl (2E)-3-methoxy-2-propenoate;ethylhydrazinewith sodium hydroxide;water at 40; pH=9.0 - 9.5; for 4 h;
Stage #2: with hydrogenchloride in water at 5; pH=3 - 4;
Steps:
139.A; 140.A
Example 139; (S)- 1 -(5-(3-(I -Ethyl- 1 H-pyrazol-5-yloxy)5-(pyridin-2-ylthio)pyridin-2-ylaminoV- 12.A- thiadiazol-3-vl )ethane- 1.2-diol hydrochloride; Step A: In IL glass beaker, ethylhydrazine oxalate (51.4 g, 342 mmol) was combined with water (300 ml). To the resulting slurry was added a 50% w/v NaOH solution (75.5 g) to
References:
WO2009/42435,2009,A1 Location in patent:Page/Page column 90-91; 94

5788-17-0
171 suppliers
$21.00/5mL
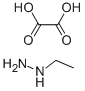
6629-60-3
98 suppliers
$52.00/5g

107296-34-4
16 suppliers
$108.00/100mg