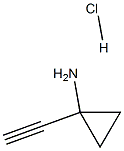
1-EthynylcyclopropanaMine hydrochloride synthesis
- Product Name:1-EthynylcyclopropanaMine hydrochloride
- CAS Number:1268810-17-8
- Molecular formula:C5H8ClN
- Molecular Weight:117.5767
1268810-08-7
17 suppliers
inquiry

1268810-17-8
47 suppliers
$65.00/100mg
Yield:-
Reaction Conditions:
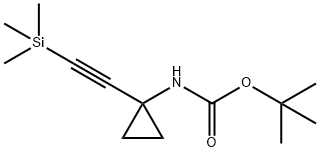
Stage #1:tert-butyl N-[1-(2-trimethylsilylethynyl)cyclopropyl]carbamate with potassium fluoride in water;N,N-dimethyl-formamide for 16 h;
Stage #2: with hydrogenchloride in 1,4-dioxane for 16 h;
Steps:
Intermediate S17-D 1 -Ethynylcyclopropanamine hydrochloride
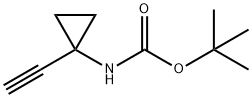
A mixture of tert-butyl N-[1 -(2-trimethylsilylethynyl)cyclopropyl]carbamate (1 .31 g, 5.17 mmol) and potassium fluoride (901 . mg, 15.51 mmol) in DMF (75 mL) and water (75 mL) was stirred for 16 h. EtOAc (200 mL) and saturated aqueous sodium bicarbonate (200 mL) were added and the mixture stirred for 5 mins. The EtOAc layer was separated and the aqueous layer extracted with EtOAc (100 mL). The combined EtOAc layers were passed through a hydrophobic frit and concentrated to dryness. The crude mixture was purified by column chromatography (hex->50:50 EtOAc:Hex) to give an oil. This was taken up in 4M HCI in dioxane (50 mL) and stirred for 16 h. The resultant white precipitate was filtered, washed with ether and dried yielding 1 -ethynylcyclopropanamine hydrochloride (830 mg, 7.06 mmol, 136%) as a white solid. 1 H NMR (300MHz, DMSO-d6) δ = 9.13 - 8.79 (m, 1 H), 8.96 (br s, 2H), 3.60 (s, 1 H), 1 .38 - 1 .28 (m, 2H), 1 .16 (d, J=2.6 Hz, 2H)
References:
CANCER RESEARCH TECHNOLOGY LIMITED;MCGONAGLE, Alison E.;JORDAN, Allan;WASZKOWYCZ, Bohdan;HUTTON, Colin;WADDELL, Ian;HITCHIN, James R.;SMITH, Kate Mary;HAMILTON, Niall M. WO2016/92326, 2016, A1 Location in patent:Page/Page column 102; 227
1268810-09-8
53 suppliers
inquiry

1268810-17-8
47 suppliers
$65.00/100mg