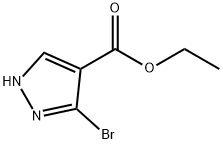
1-H-pyrazole-4-carboxylic acid,3-broMo,ethyl ester synthesis
- Product Name:1-H-pyrazole-4-carboxylic acid,3-broMo,ethyl ester
- CAS Number:1353100-91-0
- Molecular formula:C6H7BrN2O2
- Molecular Weight:219.04
6994-25-8

1353100-91-0
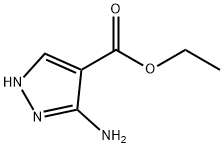
The general procedure for the synthesis of ethyl 3-bromopyrazole-4-carboxylate from 3-amino-4-ethoxycarbonylpyrazole was as follows: to a solution of ethyl 3-amino-1H-pyrazole-4-carboxylate (5.0 g, 32 mmol) and copper(II) bromide (7.2 g, 32 mmol) in acetonitrile (65 mL) was slowly added isoamyl nitrite (12 mL, 86 mmol). The reaction mixture was heated to 50 °C and stirred overnight. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched with 1N aqueous hydrochloric acid (150 mL). The mixture was extracted with ethyl acetate (3 x 100 mL). The organic phases were combined, washed with water, dried over anhydrous sodium sulfate, filtered and concentrated to afford ethyl 3-bromopyrazole-4-carboxylate as a brown oil, which was partially cured overnight under vacuum (7.1 g, 100%). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 9.78 (br.s, 1H), 8.10 (br.s, 1H), 4.33 (q, J = 7.22 Hz, 2H), 1.36 (m, 3H).

6994-25-8
409 suppliers
$5.00/1g

1353100-91-0
82 suppliers
$19.00/100mg
Yield:1353100-91-0 100%
Reaction Conditions:
with copper(ll) bromide;isopentyl nitrite in acetonitrile at 50;
Steps:
5.1
To a 0° C. solution of ethyl 3-amino-1H-pyrazole-4-carboxylate (5.0 g, 32 mmol) and copper (II) bromide (7.2 g, 32 mmol) in acetonitrile (65 mL) was slowly added isoamyl nitrite (12 mL, 86 mmol). The reaction was heated to 50° C. and stirred overnight. The reaction was cooled to room temperature and quenched with 1 N aqueous hydrochloric acid (150 mL). The mixture was extracted with ethyl acetate (3×100 mL). The combined organics were washed with water, dried over sodium sulfate, filtered, and concentrated to give the title compound as a brown oil that partially solidified under vacuum overnight (7.1 g, 100%). 1H NMR (400 MHz, CDCl3, δ): 9.78 (br. s., 1H), 8.10 (br. s., 1H), 4.33 (q, J=7.22 Hz, 2H), 1.36 (m, 3H).
References:
Pfizer Inc US2012/108619, 2012, A1 Location in patent:Page/Page column 26