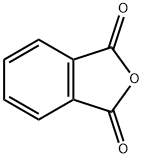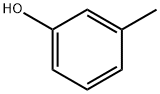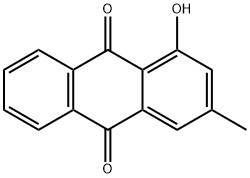
1-Hydroxy-3-methylanthracene-9,10-dione synthesis
- Product Name:1-Hydroxy-3-methylanthracene-9,10-dione
- CAS Number:2549-78-2
- Molecular formula:C15H10O3
- Molecular Weight:238.24
Yield: 49.7%
Reaction Conditions:
with aluminum (III) chloride;sodium chloride at 150 - 170; for 0.75 h;
Steps:
2.3. Synthesis of Anthraquinones
General procedure: A series of anthraquinone derivatives was prepared as described by Singh and Geetanjali [25] with slight modification.In a dry two-necked round bottom flask was placed 45g aluminum chloride (308 mmol) and 9 g of sodium chloride (155 mmol). The mixture was heated on an oil bath until melted (external temperature, 150-170oC). Phthalic anhydride(1 g, 6.75 mmol) and hydroxyl-substituted benzene (15mmol) were slowly introduced into the molten aluminum chloride and sodium chloride and heated with stirring for 45 min. Upon the completion of the reaction, the deep redmelt was carefully poured onto 500 ml ice water. About 15 ml of concentrated hydrochloric acid was added to the mixture and it was allowed to stand overnight for product precipitation. The precipitate was collected using vacuum filtration and washed with saturated sodium bicarbonate solutionto remove acidic impurities. The sample was premixed with acid-treated silica and subjected to open column chromatography or medium pressure liquid chromatography eluted with composition of n-hex: CH2Cl2 or n-hex: EtOAc to give yellow to red coloured products. The o-alkylation reactionwas accomplished using method as described earlier [26]. Ina dry round bottom flask was placed hydroxyanthraquinone(1 mmol), methyl iodide (2 mmol) and potassium carbonate(1 mmol) in 30 ml acetone. The mixture was refluxed for8-120 h. The completion of the reaction was monitored using analytical TLC. Upon the completion of the reaction, the mixture was dried. The crude product was redissolved in dichloromethane and extracted with distilled water to remove potassium carbonate residues. The organic layer was dried over sodium sulfate anhydrous and premixed with silicaprior to purification using open column chromatography or medium pressure liquid chromatography and/or preparative thin layer chromatography. Combination of n-hex: EtoAcand n-hex: CH2Cl2 were used as eluting solvents.
References:
Osman, Che Puteh;Ismail, Nor Hadiani;Widyawaruyanti, Aty;Imran, Syahrul;Tumewu, Lidya;Choo, Chee Yan;Ideris, Sharinah [Letters in drug design and discovery,2019,vol. 16,# 3,p. 353 - 363]
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2549-78-2
25 suppliers
$55.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2549-78-2
25 suppliers
$55.00/1mg
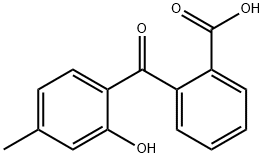
5211-78-9
0 suppliers
inquiry

2549-78-2
25 suppliers
$55.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

2549-78-2
25 suppliers
$55.00/1mg