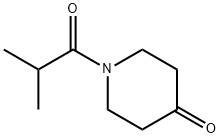
1-isobutyrylpiperidin-4-one synthesis
- Product Name:1-isobutyrylpiperidin-4-one
- CAS Number:86996-26-1
- Molecular formula:C9H15NO2
- Molecular Weight:169.22
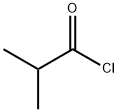
79-30-1
368 suppliers
$10.00/10g

86996-26-1
28 suppliers
$46.00/1g
Yield:86996-26-1 82%
Reaction Conditions:
Stage #1: 4-piperidone hydrochloridewith potassium carbonate in dichloromethane; for 0.0833333 h;
Stage #2: isobutyryl chloride in dichloromethane at 20; for 16 h;
Steps:
Preparation of 1-Isobutyryl -4-piperidone C9H15NO25 MW: 169.2233To a solution of 4-piperidonehydxochloride monohydrate (750 mg, 4.88 mmol) in DCM (25 ml), was added potassium carbonate (2.02 g, 14.65 mmol). After 5 minutes stirring, the addition of isobutyryl chloride (1.04 g, 9.76 mmol) proceeded at room temperature and stirring continued for a further 16 hours. The reaction was then quenched with IM NaOH (15 ml) and then extracted with DCM (3 x 20 ml). The combined organics were dried (MgSO4), filtered and concentrated in vacuo. The obtained yellow oil then underwent purification by flash chromatography (eluant; 1:1 hexane : ethyl acetate) and the relevant fractions were concentrated in vacuo to provide the title compound as a transparent oil (677 mg, 82%). 1H NMR (300 MHz, CDCl3): δ 1.12 (6H, d, J= 5.1 Hz, 2 x CH3), 2.42 (4H, t, J= 4.5 Hz, 4 x CH), 2.77-2.85 (IH, sept, J= 5.1 Hz, CH), 3.75-3.83 ppm (4H, bd, J = 23.7 Hz, 4 x CH). LCMS: M+H: 170.22 HPLC: 100% (1.960 min, isocratic 90% acetonitrile, 10% water at 1 ml/min).
References:
WO2007/3934,2007,A2 Location in patent:Page/Page column 158