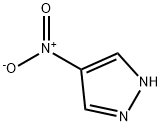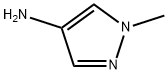
1-METHYL-1H-PYRAZOL-4-YLAMINE synthesis
- Product Name:1-METHYL-1H-PYRAZOL-4-YLAMINE
- CAS Number:69843-13-6
- Molecular formula:C4H7N3
- Molecular Weight:97.12
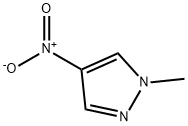
3994-50-1

69843-13-6
1-Methyl-4-nitro-1H-pyrazole (1.62 g, 12.7 mmol) was used as the raw material, which was dissolved in methanol (250 mL) and hydrogenated on an H-Cube reactor at 60 bar hydrogen pressure and 70 °C to obtain 1-methyl-1H-pyrazol-4-amine (1.23 g, 99% yield). Subsequently, 1-methyl-1H-pyrazol-4-amine (700 mg, 7.0 mmol), 3-amino-6-bromopyridinecarboxylic acid (1.86 g, 8.5 mmol), PyBop (4.12 g, 8.0 mmol), dichloromethane (30 mL), and diisopropylethylamine (3.8 mL) were added to a 100 mL round-bottomed flask and the reaction mixture was stirred at room temperature for 24 h. The reaction progress was monitored by LCMS. Upon completion of the reaction, the solvent was removed by distillation and the crude product was purified by fast chromatography (eluent: heptane/ethyl acetate). A portion of the product was further purified by reversed-phase HPLC to afford the target compound 317.The product characterization data were as follows:1H NMR (400 MHz, DMSO) δ 10.25 (s, 1H), 8.03 (s, 1H), 7.70 (s, 1H), 7.43 (d, J = 8.7 Hz, 1H), 7.19 (d, J = 8.7 Hz, 1H), 7.02 (br, 2H), 3.81 (s, 3H); MS (ESI) m/z: 296.0/298.0 [M + H]+.

3994-50-1
146 suppliers
$7.00/1g

69843-13-6
174 suppliers
$8.00/1g
Yield:69843-13-6 99%
Reaction Conditions:
with hydrogen in methanol at 70; under 45004.5 Torr;
Steps:
317
Example 317 3-amino-6-bromo-N-(1-methyl-1H-pyrazol-4-yl)picolinamide 317 1-Methyl-4-nitro-1H-pyrazole (1.62 g, 12.7 mmol) was dissolved in methanol (250 mL) and hydrogenated on H-Cube at 60 bar hydrogen pressure and 70° C. to give 1-methyl-1H-pyrazol-4-amine (1.23 g, 99%). To a 100 mL round bottom flask containing 1-methyl-1H-pyrazol-4-amine (700 mg, 7.0 mmol), 3-amino-6-bromopicolinic acid (1.86 g, 8.5 mmol) and PyBop (4.12 g, 8.0 mmol) was added methylene chloride (30 mL) and diisopropylethylamine (3.8 mL, 21.6 mmol). The reaction mixture was stirred for 24 hr at room temperature and the reaction was monitored by LCMS. Upon completion of the reaction, the solvent was distilled off and the crude material was purified via flash chromatography, heptane/ethyl acetate 0% to 100% to afford a yellow solid. A fraction of it was purified via reverse phase HPLC to afford 317. 1H NMR (400 MHz, DMSO) δ 10.25 (s, 1H), 8.03 (s, 1H), 7.70 (s, 1H), 7.43 (d, J=8.7 Hz, 1H), 7.19 (d, J=8.7 Hz, 1H), 7.02 (br, 2H), 3.81 (s, 3H). MS (ESI) m/z: 296.0/298.0 [M+H+].
References:
Wang, Xiaojing US2011/251176, 2011, A1 Location in patent:Page/Page column 160-161
15803-02-8
338 suppliers
$5.00/1g

69843-13-6
174 suppliers
$8.00/1g
54210-32-1
72 suppliers
inquiry

69843-13-6
174 suppliers
$8.00/1g