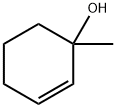
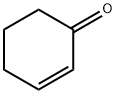
1-Methyl-2-cyclohexen-1-ol synthesis
- Product Name:1-Methyl-2-cyclohexen-1-ol
- CAS Number:23758-27-2
- Molecular formula:C7H12O
- Molecular Weight:112.17
Yield:23758-27-2 100%
Reaction Conditions:
Stage #1: cyclohexenone;methyllithiumwith lithium bromide in 2-methyltetrahydrofuran;diethyl ether at 0; for 2 h;
Stage #2: with ammonium chloride in 2-methyltetrahydrofuran;diethyl ether;water at 0;regioselective reaction;
Steps:
4.3. General procedure for LiBr mediated 1,2-addition of different organolithiums in 2-MeTHF to α,β-unsaturated compounds
General procedure: In a typical experiment a mixture of α,β-unsaturated compound (2.00 mmol, 1.0 equiv) and LiBr (3.00 mmol, 1.5 equiv) in freshly distilled 2-MeTHF (5 mL) was cooled at 0 °C. Organolithium reagents (3.00 mmol, 1.5 equiv) were then added drop-wise. After 2 h, reactions were quenched with saturated ammonium chloride aqueous solution (5 mL). After extraction with ethyl acetate (2×10 mL), desiccation over sodium sulfate, filtration, and solvent removal under reduced pressure, whenever necessary, the crude products were purified by liquid chromatography.
References:
Pace, Vittorio;Castoldi, Laura;Hoyos, Pilar;Sinisterra, José Vicente;Pregnolato, Massimo;Sánchez-Montero, Ma. José [Tetrahedron,2011,vol. 67,# 14,p. 2670 - 2675] Location in patent:supporting information; experimental part
930-68-7
267 suppliers
$9.00/1g
2999-74-8
7 suppliers
inquiry

23758-27-2
5 suppliers
inquiry
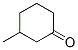
625-96-7
3 suppliers
inquiry
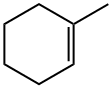
591-49-1
154 suppliers
$10.00/1g
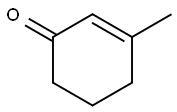
1193-18-6
182 suppliers
$6.00/1g
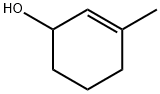
21378-21-2
61 suppliers
$63.70/1g

23758-27-2
5 suppliers
inquiry
![1-methyl-7-oxabicyclo[4.1.0]heptane](/CAS/GIF/1713-33-3.gif)
1713-33-3
57 suppliers
$28.00/250mg

930-68-7
267 suppliers
$9.00/1g
676-58-4
292 suppliers
$15.00/25ml

23758-27-2
5 suppliers
inquiry

625-96-7
3 suppliers
inquiry