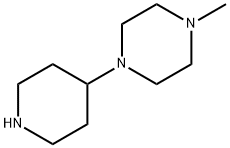
1-METHYL-4-(PIPERIDIN-4-YL)-PIPERAZINE synthesis
- Product Name:1-METHYL-4-(PIPERIDIN-4-YL)-PIPERAZINE
- CAS Number:53617-36-0
- Molecular formula:C10H21N3
- Molecular Weight:183.29
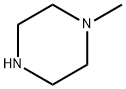
109-01-3

79099-07-3

53617-36-0
General procedure for the synthesis of 1-methyl-4-(4-piperidinyl)piperazine from N-methylpiperazine and N-tert-butoxycarbonyl-4-piperidinone: N-tert-butoxycarbonyl-4-piperidinone (1.50 g, 7.53 mmol) was dissolved in dichloromethane (25.0 mL) at 0 °C, followed by stirring of the reaction mixture for 16 h at room temperature. Upon completion of the reaction, the reaction solution was cooled to 0 °C, neutralized by addition of saturated aqueous sodium bicarbonate solution, and then extracted with dichloromethane. The organic layers were combined, dried with anhydrous sodium sulfate, filtered and the filtrate was concentrated under reduced pressure. The concentrated residue was dissolved in 1.0 N hydrochloric acid and extracted with ethyl acetate. The aqueous layer was alkalized with 48% aqueous sodium hydroxide and extracted again with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, then the filtrate was concentrated under reduced pressure. The residue was dissolved in methanol (25.0 mL), concentrated hydrochloric acid (5.0 mL) was added, and stirred at 40°C for 12 hours. After the reaction solution was concentrated and dried, the residue was dissolved in distilled water, alkalized with 48% aqueous sodium hydroxide and subsequently extracted with dichloromethane. The organic layer was dried with anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure to give 4-(1-methylpiperazin-4-yl)piperidine (0.826 g, 4.51 mmol, 60% yield) as a white solid.
Yield:53617-36-0 60%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 0 - 20; for 16 h;
Steps:
21 Synthesis of 4-(1-methylpiperazin-4-yl)piperidine
1-Methylpiperazine (0.905 g, 9.03 mmol), sodium triacetoxyborohydride (1.92 g, 9.03 mmol), and acetic acid (0.497 g, 8.28 mmol) were added to a solution of 1-tert-butoxycarbonyl-4-piperidinone (1.50 g, 7.53 mmol) in dichloromethane (25.0 mL) at 0° C., and the resulting mixture was stirred at room temperature for 16 hours. The reaction liquid was cooled to 0° C. A saturated aqueous solution of sodium hydrogencarbonate was added to the reaction liquid, and the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was dissolved in hydrochloric acid (1.0 N), and the resulting mixture was extracted with ethyl acetate. A 48% aqueous solution of sodium hydroxide was added to the aqueous layer for basification, and then the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was dissolved in methanol (25.0 mL), and concentrated hydrochloric acid (5.0 mL) was added, and then the resulting mixture was stirred at 40° C. for 12 hours. The reaction liquid was concentrated and exsiccated, and then the residue was dissolved in distilled water. A 48% aqueous solution of sodium hydroxide was added for basification, and then the resulting mixture was extracted with dichloromethane. The organic layer was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. 4-(1-Methylpiperazin-4-yl)piperidine (0.826 g, 4.51 mmol, 60%) was obtained as a white solid.
References:
TORAY INDUSTRIES, INC.;MORITA, YASUHI;IZUMIMOTO, NAOKI;ISEKI, KATSUHIKO;IWANO, SHUNSUKE;UDAGAWA, SHUJI;MIYOSHI, TOMOYA;OSADA, YUJI;KOREEDA, TETSURO;MURAKAMI, MASANORI;SHIRAKI, MOTOHIRO;TAKAHASHI, KEI;OSHIDA, KEIYU TW2016/2093, 2016, A Location in patent:Paragraph 0319

109-01-3
673 suppliers
$5.00/5 g

53617-36-0
203 suppliers
$8.00/1g

79099-07-3
0 suppliers
$5.00/5g

53617-36-0
203 suppliers
$8.00/1g
3612-20-2
446 suppliers
$6.00/10g

53617-36-0
203 suppliers
$8.00/1g