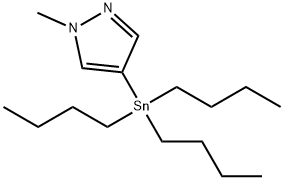
1-Methyl-4-(tributylstannyl)-1H-pyrazole synthesis
- Product Name:1-Methyl-4-(tributylstannyl)-1H-pyrazole
- CAS Number:179055-21-1
- Molecular formula:C16H32N2Sn
- Molecular Weight:371.15
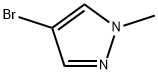
15803-02-8
338 suppliers
$5.00/1g
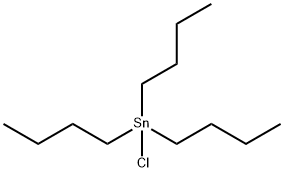
1461-22-9
218 suppliers
$10.00/10g

179055-21-1
16 suppliers
$686.17/1G
Yield:179055-21-1 36.87%
Reaction Conditions:
Stage #1: 4-bromo-1-methyl-1H-pyrazolewith n-butyllithium in tetrahydrofuran at -20 - -10; for 0.333333 h;Inert atmosphere;
Stage #2: tributyltin chloride in tetrahydrofuran at 20; for 16 h;
Steps:
STEP 1: SYNTHESIS OF COMPOUND A207-2.
n-BuLi (8.2 mL, 8.2 mmol) was added dropwise at -20/-10°C. under N2 atmosphere to a solution of compound A207-1 (1.2 g, 7.45 mmol) in THF (5 mL), and the resulting mixture was stirred at -10°C. for 20 minutes before a solution of Bu3SnCl (3.15 g, 9.69 mmol) in anhydrous THF was added. The resulting mixture was stirred at room temperature for 16 hours. The mixture was then diluted with EtOAc and washed with saturated aq. NH4Cl solution and brine, dried over Na2SO4, filtered and concentrated under vacuum. The residue was purified by column chromatography on silica gel (eluted with Petroleum Ether:EtOAc=100:0 to 25:1) to give compound A207-2 (1.02 g, 36.87% yield) as a white solid. LC/MS (ESI) m/z: 373 (M+H)+.
References:
US2021/299070,2021,A1 Location in patent:Paragraph 0427-0428