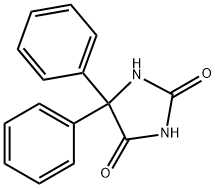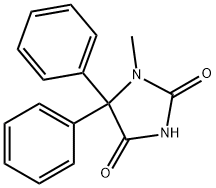
1-Methyl-5,5-diphenylimidazolidine-2,4-dione synthesis
- Product Name:1-Methyl-5,5-diphenylimidazolidine-2,4-dione
- CAS Number:6859-11-6
- Molecular formula:C16H14N2O2
- Molecular Weight:266.29
Yield:6859-11-6 79%
Reaction Conditions:
Stage #1: phenythoinwith potassium tert-butylate in tetrahydrofuran at 20; for 0.05 h;
Stage #2: methyl iodide in tetrahydrofuran at 20; for 0.0833333 h;Reagent/catalyst;Time;Solvent;
Steps:
General Procedure: Methylation of Phenytoin (1a) Using tBuOK (Table 1, run 8)
To a solution of phenytoin (1a, 100 mg, 0.40 mmol) in THF (2.0 mL), tBuOK (Aldrich, 1 M solution in THF, 0.80 mL, 0.80 mmol) was added at r.t. After 3 min, CH3I (30 μL, 0.48 mmol) in THF (0.1 mL) was added at r.t. and the mixture was stirred at r.t. for 5 min. One molar HCl (2 mL) was added and the whole was extracted with AcOEt (2×10, 1×5 mL). The combined organic layer was washed with brine (1×5 mL) and dried over Na2SO4. The solvent was evaporated in vacuo, and the crude product was purified by column chromatography (n-hexane-AcOEt 2 : 1) to give 4a as colorless solids (83 mg, 79%). 1-Methyl-5,5-diphenylimidazolidine-2,4-dione (4a) Colorless solids. mp 216.5-218.0 °C (lit13) 223-224 °C). IR (KBr) ν (cm-1) 3024, 1768, 1703. 1H-NMR (500 MHz, CDCl3) δ (ppm) 2.77 (3H, s, CH3), 7.26-7.31 (4H, m, Ar-H), 7.38-7.42 (6H, m, Ar-H), 9.31 (1H, s, NH). 13C-NMR (125 MHz, CDCl3) δ (ppm) 26.4, 75.8, 128.2, 128.8, 128.9, 136.1, 155.9, 174.1. High resolution electrospray ionization (HRESI) MS m/z 267.1130 (Calcd for C16H14N2O2: 267.1134).
References:
Shintani, Yumi;Kato, Koichi;Kawami, Masashi;Takano, Mikihisa;Kumamoto, Takuya [Chemical and Pharmaceutical Bulletin,2021,vol. 69,# 4,p. 407 - 410]
13280-16-5
38 suppliers
$45.00/10mg

6859-11-6
9 suppliers
inquiry

856-85-9
1 suppliers
inquiry

6859-11-6
9 suppliers
inquiry
2032-13-5
0 suppliers
inquiry

6859-11-6
9 suppliers
inquiry