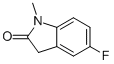
1-METHYL-5-FLUOROOXINDOLE synthesis
- Product Name:1-METHYL-5-FLUOROOXINDOLE
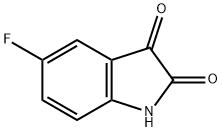
- CAS Number:41192-31-8
- Molecular formula:C9H8FNO
- Molecular Weight:165.1643
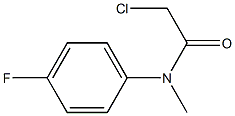
388078-30-6
6 suppliers
inquiry

41192-31-8
11 suppliers
inquiry
Yield:41192-31-8 94%
Reaction Conditions:
with Aluminum Chloride in neat (no solvent) at 170; for 4 h;
Steps:
4.3.2. 5-Fluoro-1-methylindolin-2-one (10)
Chloroacetamide 8 (0.288 g, 1.43 mmol) and anhydrous AlCl3 (0.475 g, 3.57 mmol) were heated together in a solvent free system at 170 °C for 4 h, before being slowly dripped into icewater (approx. 20 mL). The aqueous mixture was extracted with EtOAc (2 x 20 mL), and the combined organic extracts were washed with brine (15 mL) before being dried (MgSO4). The solventwas removed in vacuo to afford the target compound as a beige solid (0.221 mg, 1.34 mmol, 94%). Rf 0.41 (1:1 Hexanes:EtOAc); M.p. 105-108 °C; IR(cm-1) 1534, 1578, 1626, 1673, 2796, 2921, 2964, 3314; 1H NMR(400 MHz, DMSO-d6) 3.11 (3H, s, N-CH3), 3.57 (2H, s, oxindole-CH2),6.96 (1H, dd, J = 8.5, 4.5 Hz, H-7), 7.11 (1H, ddd, J = 9.6, 8.5, 2.6 Hz,H-6), 7.16-7.20 (1H, m, H-4); 13C NMR (100 MHz, DMSO-d6) 26.5(N-CH3), 35.9 (oxindole-CH2), 109.3 (d, JCF = 8.3 Hz, Ar-C), 112.6 (d,JCF = 25.0 Hz, Ar-C), 114.0 (d, JCF = 23.3 Hz, Ar-C), 127.1 (d,JCF = 8.9 Hz, Ar-C), 141.8 (d, JCF = 1.5 Hz, Ar-C), 158.7 (d,JCF = 236.3 Hz, Ar-C), 174.5 (C=O); HRMS calcd. for C9H7FNO (ES-)m/z 164.051167 [M-H]-, found 164.051768.
References:
Backos, Donald S.;Casalvieri, Kimberly A.;Jordan, Craig T.;Matheson, Christopher J.;Minhajuddin, Mohammed;Reigan, Philip [European Journal of Medicinal Chemistry,2020,vol. 197]
773-91-1
31 suppliers
$135.00/1g

41192-31-8
11 suppliers
inquiry
41192-39-6
1 suppliers
inquiry

41192-31-8
11 suppliers
inquiry
443-69-6
366 suppliers
$5.00/1g

41192-31-8
11 suppliers
inquiry
![2H-Indol-2-one, 5-fluoro-3-[(3,3,4,4,5,5,6,6,7,7,8,8,9,9,10,10,10-heptadecafluorodecyl)thio]-1,3-dihydro-1-methyl-](/CAS/20210305/GIF/847550-41-8.gif)
847550-41-8
0 suppliers
inquiry

41192-31-8
11 suppliers
inquiry