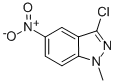
1-Methyl-5-nitro-1H-indazol-3-ylaMine synthesis
- Product Name:1-Methyl-5-nitro-1H-indazol-3-ylaMine
- CAS Number:73105-48-3
- Molecular formula:C8H8N4O2
- Molecular Weight:192.1747
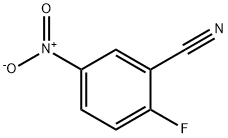
17417-09-3
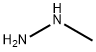
60-34-4

73105-48-3
GENERAL METHOD: A mixture of 2-fluoro-5-nitrobenzonitrile (10.0 mmol) and methylhydrazine (2.8 mL, 50.0 mmol) in ethanol (10.0 mL) was heated to reflux and reacted overnight. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently concentrated under reduced pressure. Water (10.0 mL) and ethyl acetate (20.0 mL) were added to the residue and extracted. After separation of the organic layer, it was washed sequentially with water (10.0 mL) and brine (10.0 mL), dried with anhydrous sodium sulfate, filtered, and concentrated under reduced pressure. Finally, the residue was purified by silica gel column chromatography using ethyl acetate/hexane (1:1) as eluent to afford the target product 1-methyl-5-nitro-1H-indazol-3-amine.

17417-09-3
238 suppliers
$9.00/1g

60-34-4
0 suppliers
$65.59/25g

73105-48-3
38 suppliers
$23.00/100mg
Yield:73105-48-3 94%
Reaction Conditions:
in ethanol;Reflux;regioselective reaction;
Steps:
1 General procedure for synthesis of 3-amino-1-methyl-1H-indazole 2
General procedure: A mixture of benzonitrile 1 (10.0 mmol) and methylhydrazine (2.8 mL, 50.0 mmol) in EtOH (10.0 mL) was heated to reflux overnight. The mixture was cooled to rt and then concentrated. H2O (10.0 mL) and EtOAc (20.0 mL) were added to the residue. The organic layer was washed with H2O (10.0 mL), brine (10.0 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The residue was subjected to silica-gel chromatography by using EtOAc/hexanes (1:1) as eluent to give the product 2.
References:
Liu, Han-Jun;Hung, Shiang-Fu;Chen, Chuan-Lin;Lin, Mei-Huey [Tetrahedron,2013,vol. 69,# 19,p. 3907 - 3912]
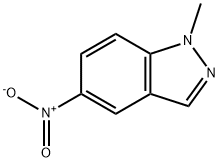
5228-49-9
145 suppliers
$15.00/250mg

73105-48-3
38 suppliers
$23.00/100mg
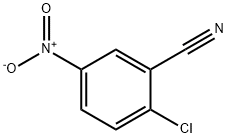
16588-02-6
213 suppliers
$7.00/1g

73105-48-3
38 suppliers
$23.00/100mg

60160-68-1
9 suppliers
inquiry

73105-48-3
38 suppliers
$23.00/100mg