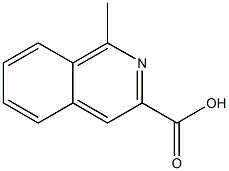
1-Methylisoquinoline-3-carboxylic acid synthesis
- Product Name:1-Methylisoquinoline-3-carboxylic acid
- CAS Number:910123-62-5
- Molecular formula:C11H9NO2
- Molecular Weight:187.1947
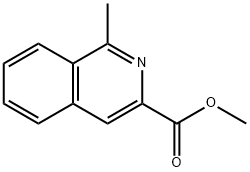
94726-23-5
0 suppliers
inquiry

910123-62-5
4 suppliers
inquiry
Yield:910123-62-5 97%
Reaction Conditions:
with sodium hydroxide in ethanol; for 0.5 h;Reflux;
Steps:
1-Methylisoquinoline-3-carboxylic acid (37).
A mixture of 38 (192 mg, 0.954 mmol) in 1NNaOH : EtOH (1 : 3, 4 mL) was refluxed for 0.5 h. After cooled down, the reaction mixture wasconcentrated and adjusted to pH = 3 with 1N HCl. The resultant mixture was dried underreduced pressure. To the residue was added EtOH (20 mL) and refluxed for 5 min. Then it wassubjected to hot filtration. The collected solid was added EtOH (20 mL) and subjected to thesame procedure twice. The combined filtration was concentrated and crystallized withMeOH/Et2O to give 173 mg white solid, in 97% yield. 1H NMR (400 MHz, CD3OD) 8.71 (s,1H), 8.54 (d, J = 8.40 Hz, 1H), 8.26 (d, J = 8.12 Hz, 1H), 8.09 (t, J = 7.30 Hz, 1H), 8.01 (t, J =7.42 Hz, 1H), 3.20 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 161.50, 138.18, 135.63, 132.52 ( 2),130.41 ( 2), 129.73, 128.56, 125.45, 19.67.
References:
Yuan, Yunyun;Elbegdorj, Orgil;Beletskaya, Irina O.;Selley, Dana E.;Zhang, Yan [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 18,p. 5045 - 5048] Location in patent:supporting information
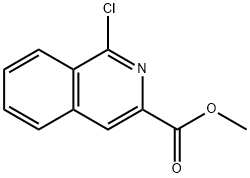
349552-70-1
35 suppliers
inquiry

910123-62-5
4 suppliers
inquiry
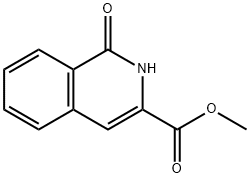
69454-42-8
30 suppliers
inquiry

910123-62-5
4 suppliers
inquiry