
(1-(methylsulfonyl)piperidin-4-yl)methanol synthesis
- Product Name:(1-(methylsulfonyl)piperidin-4-yl)methanol
- CAS Number:241134-34-9
- Molecular formula:C7H15NO3S
- Molecular Weight:193.26

217487-18-8
24 suppliers
$45.00/50mg

241134-34-9
23 suppliers
$188.00/250mg
Yield:241134-34-9 87%
Reaction Conditions:
Stage #1: 1-methanesulfonylpiperidine-4-carboxylic acid ethyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20;
Stage #2: with water in tetrahydrofuran at 0 - 10;
Stage #3: with hydrogenchloride in tetrahydrofuran;water at 0 - 20; pH=< 2; for 0.25 h;
Steps:
2
Preparation 2Preparation of (l-methanesulfonyIpiperidin-4-yl)methanol l-Methanesulfonyl-4-(ethoxycarbonyl)-piperidine (1 mol eq) was charged to a reaction vessel followed by a line wash of THF (6 rel vols). The reaction mixture was cooled to between 0 and 1O0C. A solution of lithium aluminium hydride (IM in THF, 0.75mol eq) followed by a line wash of THF (1 rel vol) was added to the vessel, keeping the temperature between 0° and 200C, and then the reaction mixture was warmed to ambient temperature and stirred until the reaction was complete. The reaction mixture was cooled to between 0 and 2°C. Purified water (1 rel vol) was then charged to the vessel maintaining the temperature between 0° to 1O0C. The pH of the reaction was adjusted to <2 by charging 5M HCl, maintaining the temperature between 0 and 1O0C. The reaction mixture was warmed to room temperature, stirred for at least 15 minutes and then the phases separated. DCM (5 rel vol) was charged to the aqueous phase, stirred for at least 15 minutes and the phases separated. The first organic (THF) phase was concentrated to approximately 3.5 rel vols by vacuum
References:
WO2007/57643,2007,A1 Location in patent:Page/Page column 6-7
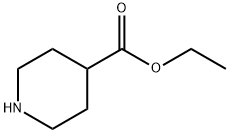
1126-09-6
477 suppliers
$15.40/25g

241134-34-9
23 suppliers
$188.00/250mg
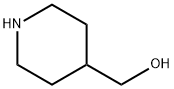
6457-49-4
361 suppliers
$18.00/25g

241134-34-9
23 suppliers
$188.00/250mg