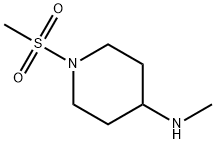
1-N-(Methylsulfonyl)-4-(aminomethyl)piperidine synthesis
- Product Name:1-N-(Methylsulfonyl)-4-(aminomethyl)piperidine
- CAS Number:438585-61-6
- Molecular formula:C7H16N2O2S
- Molecular Weight:192.28

74-89-5
0 suppliers
$13.44/25ML
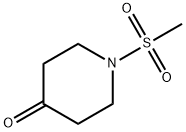
218780-53-1
85 suppliers
$13.00/250mg

438585-61-6
36 suppliers
$56.54/50mgs:
Yield:438585-61-6 62.9%
Reaction Conditions:
Stage #1: methylamine;1-(methylsulfonyl)piperidin-4-one in acetonitrile at 20; for 1 h;
Stage #2: with sodium tris(acetoxy)borohydride in acetonitrile at -10 - 20;
Stage #3: with sodium hydroxide in water;
Steps:
1.5
[0179] Compound 4 (40.00 g, 0.226 mol), CH3CN (240 ml), 40% CH3NH2 (20.4 ml, 0.263 mol) were added to a round bottom flask. The reaction mixture was stirred at room temperature for 1 hour. To another round bottom flask, NaBH(OAc)3 (60.00 g, 0.283 mol) and 120 ml of CH3CN were added. This solution was stirred at -10° C., to which the above solution was added very slowly via an additional funnel. After the addition, the reaction was allowed to warm to room temperature and stirred overnight. The reaction mixture was concentrated to a small volume, to which 1N aq. NaOH (282 ml) was added. This resulting solution was extracted with CH2Cl2 (3×500 ml) followed by extraction with toluene until no product remained in the extraction solution. The combined organic layer was dried over Na2SO4. After filtration, the solution was concentrated in vacuo to give 5 (29.00 g, 62.9%) as white solid. 1H NMR (CDCl3) δ 3.66 (m, 2H), 2.84 (m, 2H), 2.76 (s, 3H), 2.52 (m, 1H), 2.42 (s, 3H), 1.96 (m, 2H), 1.45 (m, 2H). MS m/e 193 (M+H)+
References:
US2004/34008,2004,A1 Location in patent:Page/Page column 12-13