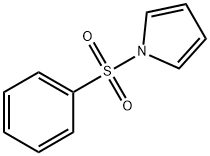
1-(PHENYLSULFONYL)PYRROLE synthesis
- Product Name:1-(PHENYLSULFONYL)PYRROLE
- CAS Number:16851-82-4
- Molecular formula:C10H9NO2S
- Molecular Weight:207.25
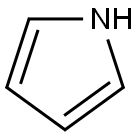
109-97-7
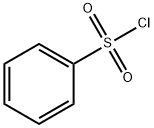
98-09-9

16851-82-4
A solution of benzenesulfonyl chloride (1.92 mL, 15.0 mmol) in toluene (15.0 mL) was slowly added dropwise to a mixed system of toluene (30.0 mL) containing pyrrole (0.69 mL, 10.0 mmol), tetrabutylammonium hydrogensulfate (0.34 g, 1.0 mmol), and a 50% aqueous sodium hydroxide solution (10.0 mL) over a period of 15 min. The reaction mixture was stirred at room temperature and the progress of the reaction was monitored by thin layer chromatography (TLC).After 3 h, TLC analysis indicated that the reaction was complete. Subsequently, the two phases of the reaction solution were separated and the organic phase was washed sequentially with water and saturated saline, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product was purified by fast column chromatography (silica gel as stationary phase and 30% dichloromethane/hexane as eluent) to afford 1.81 g (87% yield) of the target compound 1-phenylsulfonylpyrrole as a white solid. Melting point: 86-87°C; 1H NMR (300 MHz, CDCl3) δ 6.30 (m, 2H), 7.17 (m, 2H), 7.50 (m, 2H), 7.57 (m, 1H), 7.84 (m, 1H), 7.87 (m, 1H).

109-97-7
353 suppliers
$9.00/5 g

98-09-9
364 suppliers
$12.00/25 g

16851-82-4
166 suppliers
$11.00/1g
Yield:16851-82-4 99%
Reaction Conditions:
with tetra(n-butyl)ammonium hydrogensulfate;sodium hydroxide in dichloromethane;water at 20;Inert atmosphere;Cooling;
Steps:
1-(Phenylsulfonyl)-1H-pyrrole 14
To a cooled solution of aqueous sodium hydroxide (50% w/v, 20 mL, 250 mmol) and dichloromethane (20 mL) was added tetrabutylammonium hydrogen sulfate (250 mg, 750 μmol) and freshly distilled pyrrole (1.0 g, 14.9 mmol) followed by benzenesulfonyl chloride (2.1 mL, 16.4 mmol). The mixture was stirred at room temperature for 3 hours, quenched with saturated aqueous ammonium chloride (200 mL) and extracted with dichloromethane (3 x 40 mL). The combined organic extracts were washed with saturated aqueous sodium hydrogen carbonate (2 x 50 mL) and saturated aqueous sodium chloride (50 mL), dried over magnesium sulfate and concentrated to afford the desired product (3.1 g, 99%) as an off-white solid, mp: 88.5-89.3 °C; Rf: 0.47 (1:4 diethyl ether, hexane); IR (vmax (film)): 2962, 2875, 1482, 1450, 1365, 1314, 1168, 726 cm-1; 1H NMR (300 MHz, CDCl3): δ 6.30 (2H, t, J = 2.1 Hz), 7.17 (2H, t, J = 2.1 Hz), 7.44-7.55 (2H, m), 7.55-7.70 (1H, m), 7.81-7.89 (2H, m) ppm; 13C NMR (75 MHz, CDCl3): δ 113.9, 121.1, 127.0, 129.6, 134.1, 139.4 ppm.
References:
Moir, Michael;Boyd, Rochelle;Gunosewoyo, Hendra;Montgomery, Andrew P.;Connor, Mark;Kassiou, Michael [Tetrahedron Letters,2019,vol. 60,# 36,art. no. 151019] Location in patent:supporting information
696-59-3
408 suppliers
$7.00/25g
98-10-2
358 suppliers
$21.00/25g

16851-82-4
166 suppliers
$11.00/1g
25630-24-4
18 suppliers
$158.00/1g

16851-82-4
166 suppliers
$11.00/1g
16851-71-1
7 suppliers
inquiry

16851-71-1
7 suppliers
inquiry

16851-82-4
166 suppliers
$11.00/1g

696-59-3
408 suppliers
$7.00/25g

98-10-2
358 suppliers
$21.00/25g
40899-71-6
153 suppliers
$5.00/1g

16851-82-4
166 suppliers
$11.00/1g