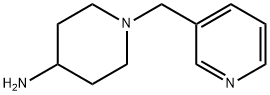
1-PYRIDIN-3-YLMETHYL-PIPERIDIN-4-YLAMINE synthesis
- Product Name:1-PYRIDIN-3-YLMETHYL-PIPERIDIN-4-YLAMINE
- CAS Number:160357-88-0
- Molecular formula:C11H17N3
- Molecular Weight:191.27
![Acetamide, 2,2,2-trifluoro-N-[1-(3-pyridinylmethyl)-4-piperidinyl]-](/CAS/20210305/GIF/431897-72-2.gif)
431897-72-2
0 suppliers
inquiry

160357-88-0
27 suppliers
$45.00/50mg
Yield:160357-88-0 11.2 g (88%)
Reaction Conditions:
with sodium borohydrid in 1,4-dioxane;ethanol;water;
Steps:
1.b 1-(3-pyridylmethyl)-4-aminopiperidine
Step b 1-(3-pyridylmethyl)-4-aminopiperidine 20.8 g (0.53 mol) of sodium borohydride are added to a solution of 19 9 (0.066 mol) of 2,2,2-trifluoro-N-[1-(3-pyridylmethyl)4-piperidyl]acetamide in 640 ml of dioxane and 64 ml of absolute ethanol, and the resulting mixture is refluxed under nitrogen for 10.5 hours. After concentrating and taking up the residue in water, the mixture is extracted with dichloromethane. The organic phase is dried over sodium sulphate and concentrated to give 11.2 g (88%) of an oil, which is used without further purification in the following step.
References:
US2004/34028,2004,A1