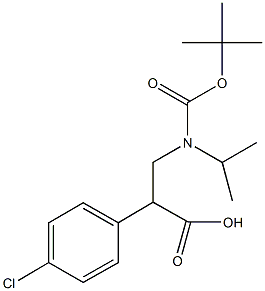
3-{[(tert-butoxy)carbonyl](propan-2-yl)amino}-2-(4-chlorophenyl)propanoic acid synthesis
- Product Name:3-{[(tert-butoxy)carbonyl](propan-2-yl)amino}-2-(4-chlorophenyl)propanoic acid
- CAS Number:1000985-05-6
- Molecular formula:C17H24ClNO4
- Molecular Weight:341.83
amino]methyl]-, methyl ester](/CAS/20210305/GIF/1000985-04-5.gif)
1000985-04-5
0 suppliers
inquiry
amino}-2-(4-chlorophenyl)propanoic acid](/CAS/GIF/1000985-05-6.gif)
1000985-05-6
8 suppliers
inquiry
Yield:1000985-05-6 60%
Reaction Conditions:
Stage #1: methyl 3-(tert-butoxycarbonyl(isopropyl)amino)-2-(4-chlorophenyl)propanoatewith lithium hydroxide;water in tetrahydrofuran; for 4 h;
Stage #2: with hydrogenchloride in water; pH=2 - 3;
Steps:
1.11
Step 11:; Methyl 2-(4-chlorophenyl)acrylate (1.00 g, 5.09 mmol) was added as a solution in 2.5 mL of THF to a stirring solution of i-PrNH2 (650 uL, 7.63 mmol) in 10 mL of THF. The reaction was allowed to stir at room temperature overnight to completion by LCMS analysis. The solvent was removed under reduced pressure to give methyl 2-(4-chlorophenyl)-3- (isopropylamino)propanoate (LCMS (APCI+) [M-Boc+H]+ 256.1, Rt: 1.97 min), which was re- dissolved in 15 mL of DCM at room temperature. The Boc2O (1.29 mL, 5.59 mmol) was added to the stirring amine via pipette followed by a catalytic amount (1 mg) of DMAP. The reaction was allowed to stir overnight to completion by LCMS and TLC analysis of the mixture. The solution was concentrated in vacuo to afford methyl 3-(tert-butoxycarbonyl(isopropyl)amino)-2-(4- chlorophenyl)propanoate as an oily residue (LCMS (APCI+) [M-Boc+H]+ 256.1, Rt: 4.13 min) which was re-dissolved in 12.0 mL of THF and 4.0 mL of water. The solution was treated with LiOH-H2O (1.07 g, 25.4 mmol) and allowed to stir for 4 hours to completion by LCMS analysis. The solution was diluted with water and washed with diethyl ether (discarded). The aqueous was treated with IM HCl solution until pH 2-3 and extracted with ethyl acetate several times. The organics were combined, washed with brine, separated, dried over MgSO4, filtered, and concentrated in vacuo to afford 3-(tert-butoxycarbonyl(isopropyl)amino)-2-(4- chlorophenyl)propanoic acid as a colorless oil (1.04 g, 60%). LCMS (APCI+) [M-Boc+H]+ 242.0.
References:
WO2008/6040,2008,A1 Location in patent:Page/Page column 78
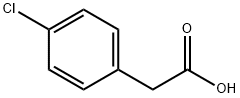
1878-66-6
444 suppliers
$6.00/25g
amino}-2-(4-chlorophenyl)propanoic acid](/CAS/GIF/1000985-05-6.gif)
1000985-05-6
8 suppliers
inquiry
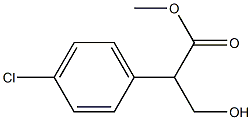
109135-06-0
0 suppliers
inquiry
amino}-2-(4-chlorophenyl)propanoic acid](/CAS/GIF/1000985-05-6.gif)
1000985-05-6
8 suppliers
inquiry
![Benzeneacetic acid, 4-chloro-α-[[(1-methylethyl)amino]methyl]-, methyl ester](/CAS/20210305/GIF/1000985-03-4.gif)
1000985-03-4
0 suppliers
inquiry
amino}-2-(4-chlorophenyl)propanoic acid](/CAS/GIF/1000985-05-6.gif)
1000985-05-6
8 suppliers
inquiry
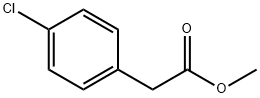
52449-43-1
187 suppliers
$6.00/5g
amino}-2-(4-chlorophenyl)propanoic acid](/CAS/GIF/1000985-05-6.gif)
1000985-05-6
8 suppliers
inquiry